Introduction to Preformulation
Preformulation is first phase in the formulation of new dosage form a new drug substance. When new drug is discovered, it needs to be restructured into the appropriate dosage form to produce desired action at a particular site. Preformulation is a study of physical and chemical properties of the drug substance alone or with the excipients, in order to develop a safe, stable and effective dosage form. In this article we will study goals, objective and physicochemical properties of drug substances.
Objectives of preformulation
- To develop the stable, safe, effective and affordable dosage form.
- To understand physicochemical parameters of new drug before mew dosage form development.
- To check compatibility of new drug
- To generate useful information for design of optimum drug delivery system.
Goals of preformulation
- To establish physicochemical properties of new drug substance.
- To establish kinetic rate profile.
- To establish compatibility with other common excipients.
- To choose correct drug substance.
Study of physicochemical characteristics of drug substances
Preformulation is important step before the formulation. Every drug substance has unique physical and chemical properties, which should be considered before the development of new safe, stable and effective dosage form. Inappropriate preformulation data can lead to poor stability of drug substance and increase overall cost and time for drug development.
Hence it is very important to study physicochemical properties of drug substance before the development of new dosage form.
Physical properties
The physical properties of new drug substance include;
- Physical form (crystal & amorphous)
- Particle size
- Particle shape
- Flow properties
- Solubility profile (pKa, pH and partition coefficient)
- polymorphism
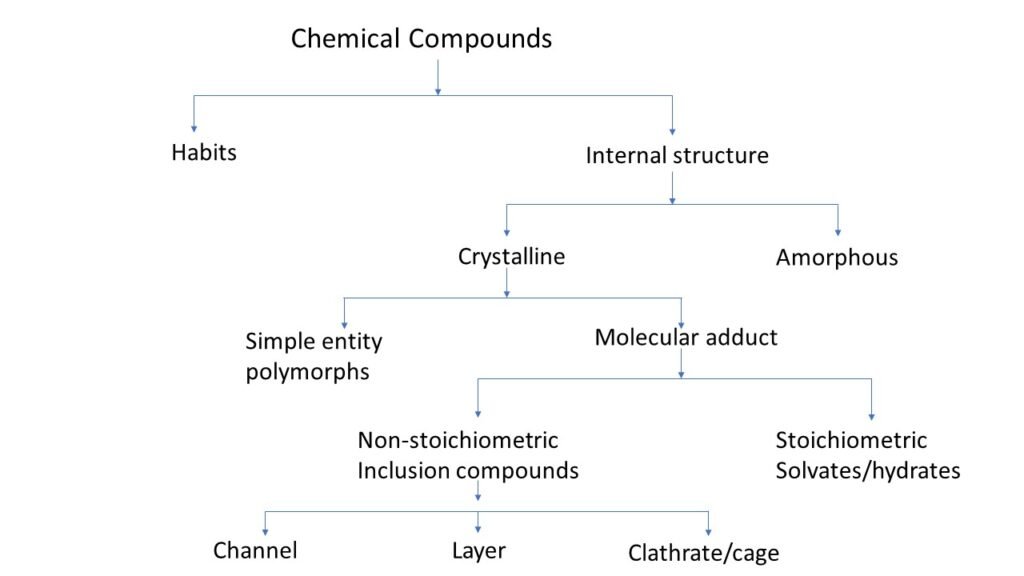
Physical form (crystalline & amorphous)
Crystal habit and internal structure affect the physicochemical property of molecule. Crystal habit is the outer appearance of chemical compound. Internal structure is the molecular arrangement within the solid.
Change in internal structure alters the habit of chemical compound. For example, conversion of sodium salt to its free acid form, changes both in internal structure and internal habit.
There are two types of chemical compound based on there internal structure one is crystalline and second is amorphous.
Difference between crystalline and amorphous forms
| Sr. no | Crystalline forms | Amorphous forms |
| 1 | Crystalline forms have fixed internal structure | Amorphous forms do not have fixed internal structure |
| 2 | More stable compare to its amorphous forms | Less stable compare to its crystalline forms |
| 3 | Has lesser thermodynamic energy as compared to its amorphous forms | Has more thermodynamic energy as compared to its crystalline forms |
| 4 | Has lesser solubility compared to its amorphous forms | Has more solubility compared to its crystalline forms |
| 5 | Crystalline forms have less tendency to change its form during storage | Amorphous forms have more tendency to change to more stable forms during storage |
Polymorphism
When a chemical substance exists more than one crystalline form accordance to its in, the various forms of substance called as polymorphs and this phenomenon is known as polymorphism.
Polymorphs are categorised as stable, metastable and unstable forms based on their thermodynamic stability.
Properties of polymorphs
| Properties of polymorphs | Stable form | Metastable form | Unstable form |
| Packing arrangement | Tightly packed | Less tightly packed | Loosely packed |
| Melting point | Highest | Moderate | Lowest |
| Dissolution rate | Lowest | Moderate | Highest |
Classification of polymorphs
Polymorphs are classified in two types;
- Enantiotropic
- Monotropic
Enantiotropic polymorph
The polymorph which changed from one form to another form on change in thermodynamic conditions called as Enantiotropic polymorph.
Monotropic polymorph
The polymorph which is unstable at all temperature and pressure conditions is called as Monotropic polymorph.
Molecular adduct
Molecular adduct is complex formed by the interaction of a drug molecule with another molecule of compound. The interaction may occur between drug and excipients, impurities or other substances used in formulation.
During the process of crystallisation some substances have a tendency to trap the solvent molecule which form lattice.
There are two types of molecular adducts.
Non-stoichiometric inclusion compound
In this crystal solvent molecules are entrapped within the crystal lattice and the number of solvent molecules is not included in stoichiometric number. Depend on the shape they are categorized in three types,
- Channel: In this solvent molecule gets trapped between continuous crystal channels.
- Layers: In this solvent molecule is get trapped between layers of crystals.
- Clathrates (cage): In this solvent molecule get trapped within the cavity of the crystal.
Stoichiometric inclusion compound
In this crystal solvent molecules are entrapped within the crystal lattice and the number of solvent molecules have stoichiometric number.
If the entrapped solvent is water, then the formed complex is called as hydrates. If the entrapped solvent is other than water then complex is called as solvates. Depend on the ratio of water molecule within a complex they are categorised as,
- Anhydrous: 1 mole compound + 0 mole water
- Semi hydrate: 1 mole compound + ½ mole water
- Mono hydrate: 1 mole compound + 1 mole water
- Dihydrate: 1 mole compound + 2 mole water
Properties of solvates/hydrates
- Anhydrous form of chemical substance has more aqueous solubility compare to its hydrates, because hydrates are already in equilibrium with water.
- Non aqueous solvates have more water solubility than the non-solvates.
Particle size
Particle size is a term used to compare the dimensions of solid particles (flecks), liquid particles (droplets), or gas particles (bubbles).
Particle size affects the various properties of chemical substance, like Dissolution rate, Solubility, Bioavailability, Lack of grittiness, Content uniformity and Penetrability.
To enhance the absorption rate of poorly soluble drug molecule, particle size should be reduced. Which also enhances bioavailability of drug.
In solid dosage forms, particle size affects the various manufacturing processes like mixing, granulation, blending, encapsulation and compression.
In liquid dosage forms, particle size affects the stability and quality of formulation. For example, small size particle can lead to unstable system and large size particle can lead to cacking.
Methods used to determine particle size
- Sieving
- Microscopy
- Sedimentation rate
- Coulter counter method
- Light energy diffraction
- Laser holography
Particle shape
Based on the shape, particles are divided into two groups,
- Symmetrical particles: The particles having specific crystal shape and easy to express in term of their diameter is known as symmetrical particles.
- Asymmetrical particles: The particle which do not have specific crystal shape known as asymmetrical particles.
Different types of particle shape
- Acicular – needle shaped
- Angular – sharp edged
- Crystalline – geometric shape
- Dendritic – branched crystalline shape
- Spherical – round shape
- Granular equidimensional irregular shape
Flow properties of powders
Flow properties of powder are very important in manufacturing processes of dosage forms. Powders may be free flowing or cohesive. Uneven powder flow can result in manufacturing defects like capping and lamination.
Evaluation parameters of powder flowability
- Carr’s compressibility index
- Hausner ratio
- Angle of repose
Factors affecting flow properties of powders
- Particle size and distribution
- Particle shape and texture
- Surface forces
Methods to improve flow properties
- Alteration of particle size and distribution
- Alteration of particle shape and texture
- Alteration of surface forces
- Addition of flow activators
Solubility profile
Solubility profile of a drug is a vital part of preformulation. It helps to obtain a suitable solution form of a drug for therapeutic action. When a drug enters in a systemic circulation, first solvent it counters is water. So, in order to show therapeutic action a drug must possess aqueous solubility.
General terms of solubility
| Term | Parts of solvent per 1 part of solute |
| Very soluble | Less than 1 part |
| Freely soluble | 1 – 10 |
| Soluble | 10 – 30 |
| Sparingly soluble | 30 – 100 |
| Slightly soluble | 100 – 1000 |
| Very insoluble | 1000 – 10000 |
| Insoluble/ practically insoluble | More than 10000 |
Solubilization
Solubilization is the process by which solubility of a poorly soluble substance is increased by the help of surfactants.
Methods to improve solubility
- Addition of cosolvent
- Change in pH
- Reduction of particle size
- Change in temperature
- Hydrotrophy
- Addition of surfactant
- Dielectric constant
- Complexation
Chemical properties
Many chemical properties of the drug substance is determined in preformulation which includes,
- Hydrolysis
- Oxidation
- Reduction
- Racemisation
- Polymerization
Hydrolysis
In this process drug molecule reacts with the water which results in splitting of chemical bonds in drug molecule. Hydrolysis process happens at a faster rate if the drug molecule contains an ester or amide functional group.
Factors affecting hydrolysis
- Presence of hydroxyl ion
- Presence of hydride ion
- Presence of divalent ion
- Solution polarity
- Drug concentration
- Heat
- Light
Oxidation
Oxidation is very common pathway for drug degradation. Most of the drug molecules will undergo oxidative degradation due to the presence of atmospheric oxygen or auto oxidation by the free radicals.
Oxidation is the process of gaining oxygen and loosing of hydrogen and/or electrons.
Factors affecting oxidation process
- Oxygen concentration
- Light
- Temperature
- Hydrogen and hydroxyl ion
Reduction
Reduction is the process of loosing an oxygen and gaining of hydrogen and/or electron.
Factors affecting reduction
- Light
- Temperature
- Hydrogen and hydroxyl ion
- Presence of other reactants
- Solvent
Racemisation
Racemisation is the process of conversion of optically active (racemic) compound into optically inactive compound. Racemisation results in a mixture which contains 1:1 molar ratio of enantiomers. This mixture is referred as racemic mixture. This mixture contains equal amount of dextrorotatory (+) and laevorotatory (-) enantiomers.
The interconversion of one isomer to another alters its pharmacokinetic properties which affects the pharmacological and toxicological effects.
Polymerisation
Polymerisation is the process in which small molecules called monomer reacts together in a chemical reaction to produce a large chainlike or network molecule called polymer. For example, darkening of glucose is due to polymerisation.
Conclusion
Preformulation is a study of physical and chemical properties of the new drug substance alone or with the excipients, in order to develop a safe, stable and effective dosage form.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “Preformulation- Goals, Objective and Physicochemical properties of drug substance”