Alkanes
Alkanes are the saturated organic compounds which consist of single bonded carbon and hydrogen atoms. That means all atoms share only one pair of electrons with each other. The general formula for alkanes is CnH2n. Where, n is the number of atoms of carbon in their chemical structure. The simplest alkane is the methane, in which four hydrogen atoms are bonded together to carbon atom via single bonds. So, the four valance electrons of carbon will bond with one valance electron of each hydrogen atom. Some examples of alkanes are, methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), etc. In this article we will see halogenation of alkanes, SP3 and SP2 hybridization.
Physical properties
Color and Odor
Alkanes are like the invisible ninjas of the chemical world. They have no color and no distinct odor. You won’t find them shouting, “Hey, look at me!” at any party.
Density and Solubility
- Lighter Than Water: Alkanes are lighter than water. Their densities typically hover around 0.7 g/mL for moderate carbon chains. Imagine them doing a graceful float in a glass of water.
- Solubility: Alkanes are nonpolar molecules, which means they don’t play well with water (a polar solvent). But they’re chummy with nonpolar and slightly polar solvents. So, they dissolve happily in organic solvents like a secret handshake.
Melting and Boiling Points
- Short-chain alkanes (think methane, ethane) are like summer breezes—they melt and boil at low temperatures.
- As we add more carbon atoms to the chain, the melting and boiling points rise. Hexane (C₆H₁₄) is like the cool autumn—it’s a liquid.
- Long-chain alkanes are winter warriors—they solidify into solids. Think of them as the snowflakes of the hydrocarbon world.
Comparison to Water
Alkanes are rebels—most have densities less than 1.0 g/mL. That’s why oil and grease don’t mix with water; they prefer to float on the surface, thumbing their hydrocarbon noses at H₂O.
| Molecular Name | Formula | Melting Point (°C) | Boiling Point (°C) | Physical State (at 20°C) |
| Methane | CH₄ | -182 | -164 | Gas |
| Ethane | C₂H₆ | -183 | -89 | Gas |
| Propane | C₃H₈ | -190 | -42 | Gas |
| Butane | C₄H₁₀ | -138 | -1 | Gas |
| Pentane | C₅H₁₂ | -130 | 36 | Liquid |
| Hexane | C₆H₁₄ | -95 | 69 | Liquid |
| Octane | C₈H₁₈ | -57 | 125 | Liquid |
| Decane | C₁₀H₂₂ | -30 | 174 | Liquid |
Halogenation of alkanes
Halogenation is a chemical process where one or more hydrogen atoms in an organic compound (specifically, alkanes) are replaced by halogen atoms—typically fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). Unlike the complex transformations seen in combustion, halogenation appears deceptively simple: it involves breaking a C-H bond and forming a new C-X bond (where X represents the halogen).
Chlorination of Methane
Let’s take methane (CH₄) as our guinea pig. When chlorine gas (Cl₂) encounters methane, a fascinating dance begins:
A C-H bond in methane breaks, and a new C-Cl bond forms.
The result, Methyl chloride (CH₃Cl) and hydrogen chloride (HCl) are born.
The equation looks like this:
CH₄ + Cl₂ + energy → CH₃Cl + HCl
Mixture of Products
- Here’s the twist: All hydrogen atoms in an alkane can undergo substitution. So, halogenation often results in a mix of products.
- For methane, if we have an excess of chlorine, we get chloroform (CHCl₃) and carbon tetrachloride (CCl₄) alongside methyl chloride.
- The relative amounts of these products depend on the reactant proportions.
Empirical Considerations
- Reactivity Order of Halogens: It goes like this—F₂ > Cl₂ > Br₂ > I₂. Fluorine is explosively reactive, so we usually stick to chlorine and bromine.
- Energy Input: Halogenations are exothermic. Heat or light (usually visible light) initiates the reaction. Each absorbed photon sets thousands of molecules grooving.
- Phase and Radical Initiators: Halogenations can happen in gas or liquid phases. Oxygen inhibits gas-phase chlorinations, while radical initiators (like peroxides) facilitate liquid-phase reactions.
Mechanism
- Picture a chain reaction involving neutral intermediates—free radicals or atoms.
- The weakest bond in the reactants is the halogen-halogen bond (Cl-Cl or Br-Br). Heat or light cleaves this bond.
- Fun fact: Both chlorine and bromine absorb visible light (which is why they’re colored).
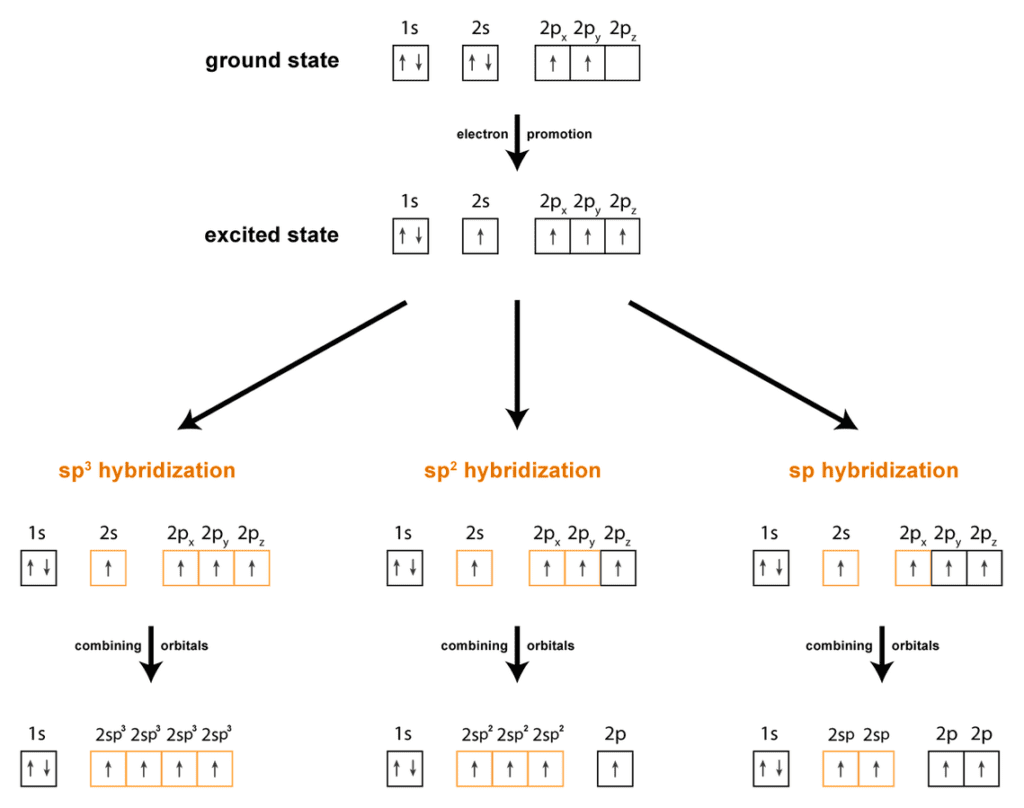
SP3 hybridization in alkanes
SP³ hybridization provides the geometric foundation for the structure of alkanes.
Hybridization Basics
- When carbon atoms bond in organic compounds, they often undergo hybridization. This process involves mixing atomic orbitals to form new hybrid orbitals.
- In the case of sp³ hybridization, one s orbital and three p orbitals of a carbon atom combine. Four new equivalent hybrid orbitals—sp³ orbitals are formed by this combination.
Geometry and Arrangement
- These sp³ hybrid orbitals arrange themselves tetrahedrally around the carbon atom. The bond angles in this arrangement are approximately 109.5 degrees.
- Imagine a methane (CH₄) molecule: Each carbon atom contributes one s orbital and three p orbitals. These combine to form four sp³ hybrid orbitals.
- The tetrahedral arrangement ensures that the carbon atom can form four sigma (σ) bonds with other atoms.
Methane (CH₄)
Methane is a classic example of sp³ hybridization. Here’s how it works:
- Carbon has one 2s orbital and three 2p orbitals.
- These hybridize to create four sp³ orbitals.
- Each sp³ orbital overlaps with a hydrogen 1s orbital, resulting in four sigma bonds.
- The methane molecule has a tetrahedral shape, with the carbon at the center and the four hydrogen atoms at the corners of the tetrahedron.
- The bond angles between the H-C-H bonds are approximately 109.5°.
- Each sp³ orbital overlaps with a hydrogen 1s orbital, resulting in four sigma (σ) bonds.
- The tetrahedral arrangement ensures structural stability and allows methane to form four identical sigma bonds.
Other Alkanes
- All carbon atoms in alkanes (such as ethane, propane, butane, and beyond) are also sp³ hybridized.
- This hybridization allows carbon atoms to form four identical sigma bonds, maintaining a tetrahedral geometry.
- So, whether it’s a simple alkane or a complex hydrocarbon chain, sp³ hybridization keeps things balanced.
Why It Matters
- Alkanes serve as the backbone of many organic molecules. Their stable tetrahedral arrangement ensures structural integrity.
- Remember, sp³ hybridization isn’t limited to carbon—it occurs in other atoms as well, whenever they need to form four sigma bonds.
Paraffins
Paraffins, also known as alkanes, belong to a family of saturated hydrocarbons. Their general formula is CₙH₂ₙ₊₂, where n represents the number of carbon atoms. Each paraffin molecule consists of carbon (C) and hydrogen (H) atoms, connected by single bonds. Benzene, a parent compound of numerous aromatic compounds, is derived from paraffins.
Variability in Properties
Paraffins exhibit a fascinating range of properties based on their carbon chain length:
- Gaseous Paraffins: Those with fewer than 5 carbon atoms per molecule are usually gaseous at room temperature. Think of methane (CH₄), the simplest paraffin—it’s the main component of natural gas.
- Liquid Paraffins: Those with 5 to 15 carbon atoms are typically liquids. Examples include hexane (C₆H₁₄) and octane (C₈H₁₈).
- Solid Paraffins: Straight-chain paraffins with more than 15 carbon atoms per molecule are solids. You’ll find them in waxes and petroleum jelly.
Branching Matters
- Branched-chain paraffins have a higher octane number (a measure of gasoline quality) than straight-chain ones.
- In gasoline, we prefer the more desirable constituents—those with higher octane numbers.
Uses of Paraffins
Paraffin Wax
- Candles: Paraffin wax is widely used in candle making. It provides a clean-burning, long-lasting flame and is the go-to material for most commercial candles.
- Cosmetics: It appears in cosmetics—think lip balms, lotions, and creams. Paraffin helps lock in moisture and provides a protective barrier for the skin.
- Electrical Insulation: Paraffin wax acts as an insulator in electrical components, preventing short circuits and ensuring safety.
Liquid Paraffin (Mineral Oil)
- Medicines: Highly refined liquid paraffin (also known as mineral oil) is used in pharmaceuticals. It serves as a mild laxative and is also found in some skin ointments.
- Cosmetics: Liquid paraffin appears in various cosmetic products, including makeup removers and baby oils.
- Industrial Applications: It’s used as a lubricant, especially in machinery and equipment where oil contamination is a concern.
Alkanes
- Chemical Solvent: Alkanes (including paraffins) serve as chemical solvents. They dissolve other nonpolar substances.
- Plastics: Alkanes are building blocks for plastics. They contribute to the production of polymers like polyethylene and polypropylene.
- Fuel: Paraffins are used as fuels in various contexts:
- Jet Engines and Rockets: They fuel aircraft and space exploration.
- Diesel Engines: Paraffin is a component in diesel fuel.
- Tractor Engines: It’s used in tractor vaporizing oil.
Other Uses
- Extracting Perfumes: Paraffin helps extract fragrances from flowers.
- Wood Waterproofing: It provides a waterproof coating for wood.
- Matches: In wood and paper matches, paraffin supplies an easily vaporized hydrocarbon fuel for ignition.
Alkenes
An alkene (also known as an olefin) is a hydrocarbon that contains at least one carbon-carbon double bond. These double bonds can be either internal (somewhere in the middle of the carbon chain) or terminal (at the end of the chain). Terminal alkenes are also charmingly called α-olefins. Alkenes are like the cool kids at the party—they’re unsaturated and more reactive than their saturated cousins, the alkanes. That double bond? It’s a hotbed for chemical reactions. Alkenes love to play matchmaker with other molecules, inviting them to add to their C=C bond. Think of it as a molecular dance floor where atoms waltz in and form new bonds.
Stability of alkenes
Lower Energy, More Stability
- Imagine molecules at a cosmic dance party. The ones with lower energy are the cool cats—they’re more stable.
- Alkenes, those hydrocarbon daredevils with carbon-carbon double bonds, also have their energy levels. The lower, the better!
- So, why are more substituted alkenes (the ones with extra atoms or groups hanging around) like VIPs at this party? It’s all about hyperconjugation. Think of it as molecular teamwork: Neighboring carbon-hydrogen (C-H) bonds lend their electrons to stabilize the double bond. Teamwork makes the alkene dream work!
- These well-protected alkenes have a lower heat of hydrogenation (ΔH°hydrog)—the energy released when you add hydrogen gas (H₂) to convert them into alkanes. Less energy, more stability!
The Trans Isomers
Picture two alkenes:
- cis-CH₃CH=CHCH₃: It’s like two dancers holding hands on the same side of the dance floor.
- trans-CH₃CH=CHCH₃: Here, they’re on opposite sides, doing a graceful pas de deux.
Both have similar heats of hydrogenation (around -120 kJ/mol). Because the trans alkene’s double bond is more stable with its orbital arrangement. Chemistry, always picky about dance partners!
The Orbital Overlap
- Bond strength depends on how efficiently orbitals overlap. S orbitals (those spherical ones) overlap better than p orbitals (those dumbbell-shaped ones).
- Hybrid orbitals (like sp² and sp³) join the party. The more s character they have, the better they groove. An sp² orbital (33% s character) outshines an sp³ orbital (only 25% s character).
- So, sp²-sp³ bonds (like in alkenes) are stronger than sp³-sp³ bonds (like in alkanes). It’s like comparing a solid handshake to a half-hearted high-five!
The Mystery of Substitution
It is believed that stability of alkene is increases with more substitution. Possible reasons are explained below.
- Hypothesis 1: Crowded Dance Floor: More substituents mean more steric hindrance. It’s like having too many dancers—some bump into each other, making the whole thing more stable.
- Hypothesis 2: Electron Density Party: Extra substituents mean more electron density around the double bond. Electrons love a good party, and they stabilize the bond.
SP2 hybridization of alkenes
- Picture an ethene molecule (H₂C=CH₂)—the simplest alkene. Each carbon atom in ethene wants eight valence electrons (just like a contented cat in a sunbeam). It’s the noble gas configuration craving. To achieve this, carbon undergoes sp² hybridization:
- One s orbital and two p orbitals combine.
- Result: Three sp² hybrid orbitals.
- Carbon’s three sp² hybrid orbitals arrange themselves in a trigonal planar configuration.
Trigonal Planar Shape
Ethene’s carbon atoms arrange themselves in a trigonal planar structure. Imagine a ‘peace’ sign:
- Central carbon.
- Three partners (hydrogens) on the same plane.
Each carbon forms three sigma (σ) bonds with its partners. Because sp² hybridization involves three orbitals. Carbon’s minimalist move—no need for all four.
Orbital Overlap Magic
- The sp² hybridization secret: Efficient orbital overlap.
- These hybrid orbitals (part s, part p) allow carbon to form strong bonds.
- Ethene’s double bond? It’s like synchronized swimmers—graceful and stable.
Why Sp²?
- Carbon could’ve hybridized all four outer orbitals (s and three p’s), but it’s a minimalist.
- It only needs three for the stable double bond.
- So, it uses one s orbital and two p orbitals, leaving the fourth p orbital unchanged.
- Result: sp² hybridization—made from one s and two p orbitals.
| Feature | sp² Hybridization | sp³ Hybridization |
| Hybrid Orbitals | 3 sp² orbitals | 4 sp³ orbitals |
| Geometry | Trigonal planar | Tetrahedral |
| Bond Angles | 120° | 109.5° |
| σ Bonds | 3 | 4 |
| π Bonds | 1 (due to the unhybridized p orbital) | None (all orbitals are used for σ bonding) |
| Examples | Alkenes like ethene (C₂H₄) | Alkanes like methane (CH₄) |
| Electron Density | Concentrated above and below the trigonal plane | Evenly distributed around the carbon atom |
| Reactivity | More reactive due to π bond presence (e.g., in electrophilic addition reactions) | Generally less reactive, undergoes substitution and elimination reactions |
Summary
Alkanes, the unadorned hydrocarbons, form the backbone of organic chemistry. Their simple, saturated structures—think methane, ethane, and beyond—make them reliable workhorses in fuels, waxes, and everyday products. Now, imagine carbon atoms rearranging their orbitals into tetrahedral shapes—that’s sp³ hybridization. It allows alkanes to form four sigma bonds, maintaining stability and diverse structures. But wait, when halogens (like chlorine or bromine) replace hydrogen atoms in alkanes, we get alkyl halides. These compounds play crucial roles in pharmaceuticals, solvents, and even rocket fuel. Lastly, meet the paraffins: versatile hydrocarbons that include paraffin wax (for candles and cosmetics) and liquid paraffin (used in medicines and electrical insulation). They’re ubiquitous—from skincare routines to space exploration.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us
