Electrochemical methods
Electrochemical methods in pharmaceutical analysis are powerful techniques that utilize the electrical properties of substances to determine their concentration or analyse their electrochemical behaviour. These methods often offer advantages over other analytical methods, such as a high degree of accuracy, precision, selectivity, and also low cost of operation. These methods are based on redox reactions depending on electron transfer pathways. Therefore, there is a close relationship between electrochemical and biological reactions. Electrochemical methods have been used for various applications in pharmaceutical analysis, such as biomarker detection, drug detection, metabolite determination, oxidative stress, and electrochemical therapy.
Three main techniques of electrochemical methods are widely used in pharmaceutical sciences, which includes, conductometry, potentiometry and polarography.
Conductometry
Conductometry is an analytical chemistry technique that measures the electrolytic conductivity of a solution to monitor chemical reactions. It’s used in conductometric titration, a quantitative analysis method to determine the concentration of an analyte. The technique is based on the measurement of electrolytic conductance, which is the movement of ions in a solution. It’s particularly useful in titrating-colored solutions or homogeneous suspensions where normal chemical indicators can’t be used. Conductometry has been developed since the 18th century and continues to be a valuable tool in analytical chemistry.
Conductivity cell
A conductivity cell is a device used in conductometry, an analytical method that measures the electrolytic conductivity of a solution. It typically consists of two electrodes, often made of platinum, placed in a solution. The electrodes have a known cross-sectional area ‘A’ and are separated by a known distance ‘l’.
The conductivity of the solution is determined by measuring the resistance between these two electrodes. The resistance of the solution can be given by the equation:
R = ρ(l/A)
Where,
- R is the resistance,
- ρ is the resistivity of the solution,
- l is the distance between the electrodes, and
- A is the cross-sectional area of the electrodes.
The quantity (l/A) is called the cell constant and is denoted by the symbol G.
There are different types of conductivity cells, including 2-pole cells, 3-pole cells, 4-pole cells, platinised cells, and flow-through cells. The choice of cell depends on the specific requirements of the measurement.
Conductometric titrations
Conductometric titration is a quantitative analysis method used in laboratories to determine the concentration of an analyte in a mixture. This method involves the continuous addition of a reactant to a reaction mixture and monitoring the corresponding change in its electrolytic conductivity.
Here’s a brief explanation of the process.
Principle
During a titration process, one ion is replaced with another, and the difference in the ionic conductivities of these ions directly impacts the overall electrolytic conductivity of the solution. The conductance values vary among cations and anions and depend on the occurrence of a chemical reaction in the electrolyte solution.
Theory
The end-point of the titration process can be determined by measuring conductivity. In a neutralization reaction between an acid and a base, adding the base initially lowers the solution’s conductivity because the H+ ions are replaced by the cationic part of the base. Once the equivalence point is reached, the concentration of the ionic entities will increase, thus increasing the conductance of the solution. Therefore, when plotting the conductance values graphically, two straight lines with opposite slopes will be obtained. The point where these two lines intersect is the equivalence point.
Process
For the conductometric titration of an acid with a base, the general process is as follows: Dilute 10 ml of the acid with approximately 100 ml of distilled water to minimize the changes in conductance caused by the addition of the base.
Example
Sure, let’s consider the conductometric titration of hydrochloric acid (HCl) with sodium hydroxide (NaOH). The chemical reaction equation is as follows.
HCl + NaOH → NaCl + H2O
In this reaction, hydrochloric acid, a strong acid, completely dissociates in water to form H+ and Cl- ions. Similarly, sodium hydroxide, a strong base, also dissociates completely in water to form Na+ and OH- ions. Once all the H+ ions have reacted and the equivalence point is reached, any additional NaOH added will increase the concentration of Na+ and OH- ions in the solution, thus increasing its conductivity.
Conductometric titration is especially useful in titrating homogeneous suspensions or colored solutions, which cannot be done with normal chemical indicators. It’s a powerful tool in analytical chemistry, providing valuable information about the concentration and behavior of ions in a solution.
Applications
Conductometry has a wide range of applications in various fields.
- Chemical Analysis: Conductometry analysis is widely used for the quantitative determination of the concentration of ions in a solution. It is particularly useful in the analysis of ionic compounds, such as acids, bases, and salts.
- Conductometric Titrations: Conductometry is used to monitor the progress of chemical reactions. Conductometric titration involves the continuous addition of a reactant to a reaction mixture and the documentation of the corresponding change in the electrolytic conductivity of the reaction mixture.
- Purity of Water: The determination of the purity of water can be carried out by conductimetr.
- Salinity of Sea Water: Determination of the salinity of sea water can be done by conductometry.
- Ionic Reactions: It can be used to determine chemical equilibrium in ionic reactions.
- Quantitative Analysis: Conductometric titration can be used in the quantitative analysis of compounds.
- Alkalinity of Fresh Water: The alkalinity of fresh water can be checked by this method.
- Determination of Solubility and Ksp: Conductometry can be used to determine the solubility and Ksp of sparingly soluble compounds.
- Ionization of Complex Compounds: It can be used to determine the mode of ionization of complex compounds.
- Salt Analysis: Conductometry can be used for salt analysis.
Potentiometry
Potentiometry is an electroanalytical method that measures the electrical potential between two electrodes immersed in a solution to be analysed. It involves the measurement of the potential of an electrochemical cell under static conditions. Because no current, or only a negligible current, flows through the electrochemical cell, its composition remains unchanged. This makes potentiometry a useful quantitative method for determining the concentration of a solute in solution. The potential difference measured is a function of the concentration of certain ions in the solution, making potentiometry applicable to a diverse array of analytes.
Reference electrodes
Reference Electrodes are electrodes that have a stable and well-known electrode potential. The overall chemical reaction taking place in a cell is made up of two independent half-reactions, which describe chemical changes at the two electrodes. The reference electrode is standardized with constant (buffered or saturated) concentrations of each participant of the redox reaction. This makes the reference electrode insensitive to the composition of the solution. Common examples of reference electrodes include the Standard Hydrogen Electrode (SHE), Saturated Calomel Electrode (SCE), and Silver-Silver Chloride Electrode (Ag/AgCl).
Standard hydrogen electrode (SHE)
The Standard Hydrogen Electrode (SHE) is a reference electrode used for measuring the standard electrode potential of half-cells. Its standard electrode potential is declared to be 0 at a temperature of 298K.
Construction
- A platinum electrode is covered in finely powdered platinum black, also known as a platinized platinum electrode.
- The electrode is immersed in a solution of acid having an H+ molarity of 1 mole per cubic decimete.
- Pure hydrogen gas at 1atm is bubbled through the solution.
- A hydroseal is used to prevent the interference of oxygen.
- The other half-cell of the entire Galvanic cell must be attached to the Standard Hydrogen Electrode through a reservoir in order to create an ionically conductive path.
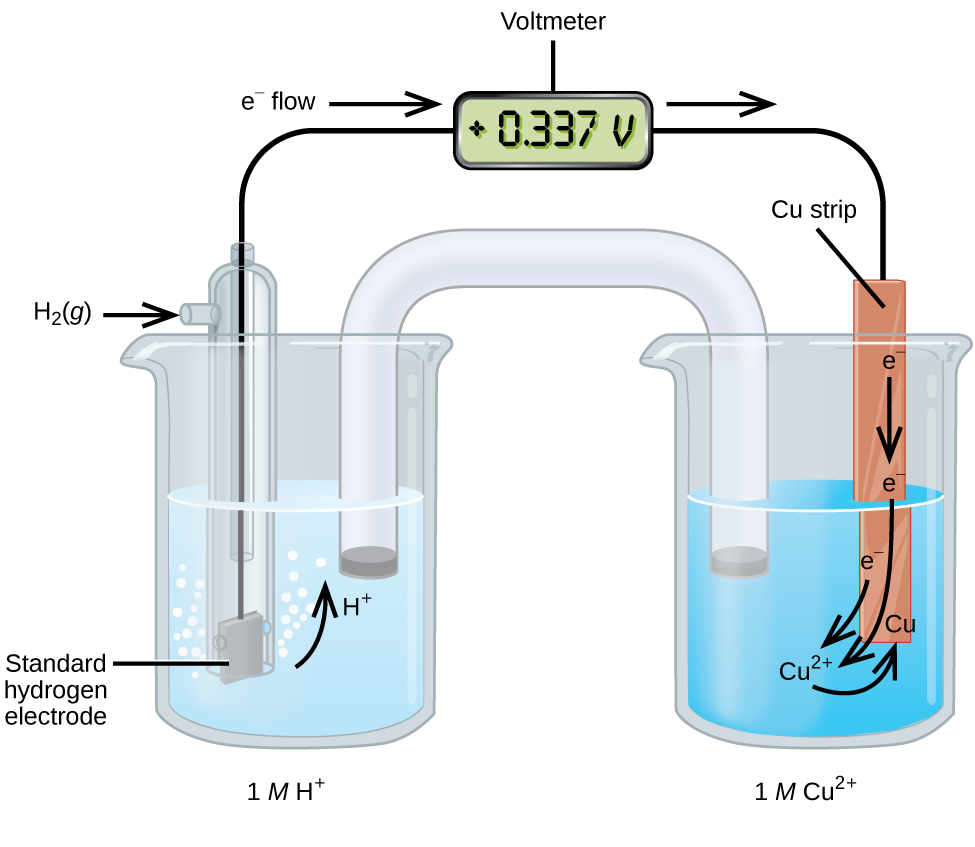
Working
- The half-cell reaction of SHE can be written as, 2H+(aq)+2e−→H2(g)
- The platinum black foil absorbs or adsorbs hydrogen gas and 1M HCl gives 1M H+ ions.
- A redox reaction takes place.
- The platinized platinum surface has a very high adsorption activity.
- Therefore, this surface must be protected from atmospheric oxygen as well as from organic substance.
- Substances such as arsenic and sulphur compounds can deactivate or poison the catalyst.
- SHE can be used as an anode half-cell or cathode half-cell depending on the requirement according to another half-cell.
- When a standard hydrogen electrode is attached as an anode half-cell then oxidation occurs on it.
- Reaction is given below.
H2 → 2H+ + 2e−
Silver chloride electrode
The Silver-Silver Chloride (Ag/AgCl) Electrode is a commonly used reference electrode in electrochemical measurements.
Construction
- The electrode consists of a metallic silver wire (Ag) coated with a thin layer of silver chloride (AgCl).
- This coating can be achieved physically by dipping the wire in molten silver chloride, chemically by electroplating the wire in concentrated hydrochloric acid (HCl), or electrochemically by oxidising the silver at an anode in a chloride solutio.
- A porous or fibrous filter located at/near the tip of the reference electrode allows to establish a liquid contact between the solution to be measured and the electrolyte solution in equilibrium with the silver chloride (AgCl) coating the Ag surface.
Working
- The electrode functions as a reversible redox electrode.
- The equilibrium is between the solid silver metal (Ag) and its solid salt—silver chloride (AgCl) in a chloride solution of a given concentration.
- The corresponding half-reaction can be presented as follows AgCl(s)+e−→Ag(s)+Cl−
- This reaction is a reversible reaction and is characterized by fast electrode kinetics, meaning that a sufficiently high current can be passed through the electrode with 100% efficiency of the redox reaction.
- The Nernst equation shows the dependence of the potential of the silver-silver (I) chloride electrode on the activity or effective concentration of chloride-ions.
- The standard electrode potential E0 against standard hydrogen electrode (SHE) is 0.230 V ± 10 mV.
Calomel electrode
The Calomel Electrode is a secondary reference electrode used in electrochemical measurements.
Construction
- The electrode consists of a glass tube with a bent side tube.
- Pure mercury is placed at the bottom of the tube.
- The mercury is covered with a paste of mercury and mercurous chloride (calomel).
- The remaining portion of the cell is filled with a solution of potassium chloride (KCl) of a definite concentration.
- A platinum wire sealed into a glass tube is dipped into the mercury layer to provide external electrical contact.
- The side tube is used for making electrical contact with a salt bridge.
Working
- The potential of the calomel electrode depends upon the concentration of the chloride ions in the solution.
- If the electrode is a saturated calomel electrode (SCE), some crystals of KCl are placed over the Hg−Hg2Cl2 paste to keep the solution saturate.
- The electrode can serve as an anode or cathode depending on the nature of another electrode of the cell.
- If the electrode serves as an anode, the half reaction that occurs on it will be oxidation. Mercury is first oxidised to mercuric ions, and the chloride ions supplied by KCl solution combine with mercuric ions [Hg2+2] to form insoluble mercurous chloride. The overall reaction is: 2Hg(l)+2Cl−(aq)→Hg2Cl2(s)+2e−
- If the electrode is a cathode, the half reaction that occurs on it will be reduction. The overall reaction is: Hg2Cl2(s)+2e−→2Hg(l)+2Cl−(aq)
- The potential of the calomel electrode decreases with an increase in the concentration of chloride ions at a given temperature. Thus, the electrode is reversible with respect to the concentration of chloride ions.
Indicator electrodes
Indicator Electrodes, also known as working electrodes, respond to changes in the analyte concentration or potential during an electrochemical reaction. They generate the signal that is measured and analysed. The potential of the indicator electrode is proportional to the analyte’s activity.
Metal and glass electrodes
Metal Electrodes are specific metal electrodes used as the indicator electrode in an electrochemical measurement. They are paired with a reference electrode and can be used to determine the concentrations of targeted analytes in a solution. There are two kinds of metal indicator electrodes.
- Electrodes of the first kind: The electrode potential responds directly in relationship to the concentration of the metal ion of interest.
- Electrodes of the second kind: The metal ion is in equilibrium with the target analyte, that equilibrium influences the availability of the metal ion to interact with the electrode.
Glass Electrodes are a type of ion-selective electrodes, that belong to the group of electrodes with a non-crystalline solid membrane. They are commonly used in potentiometric analysis to measure the potential difference between two solutions. These electrodes consist of a thin-walled glass tube filled with an electrolyte solution and a reference electrode immersed in the electrolyte. The potential developed at the membrane is the result of either an ion exchange process or an ion transport process occurring at each interface between the membrane and solution. The selectivity of the ISE is determined by the composition of the membrane. Ideally, the membrane allows the uptake of only one specific ion into it. The analyte ion may be a cation or an anion.
Methods to determine end point of potentiometric titration
There are several methods to determine the endpoint of a potentiometric titration.
- Visual Observation: This is the simplest way to determine the endpoint by visually observing the solution. The solution changes color at the endpoint due to the addition of an indicator.
- pH Measurement: In some titrations, the pH of the solution changes abruptly at the endpoint. The potential difference between the reference and indicator electrodes is measured in conditions where a thermodynamic equilibrium is maintained.
- Conductivity Measurement: Conductivity measurements can be used to determine the endpoint of some types of titrations.
- Direct Plot Method: This involves a direct plot of potential as a function of reagent volume. The midpoint in the steeply rising portion of the curve is estimated visually and taken as the end point.
- Derivative Method: This approach to end-point detection is to plot the derivative curves.
- Volumetric Method: This involves measuring the potential between two electrodes (reference and indicator) concerning the volume of reagent added.
These methods are applicable to various types of potentiometric titrations, including acid-base titration, redox titration, complexometric titration, and precipitation titration.
Applications
Potentiometry has a wide range of applications in various fields.
- Clinical Chemistry: It is used to determine electrolyte levels in clinical samples. For example, pH, conductivity, ion selective electrodes (ISEs, Cl-, Ca2+,HCN, SO2, NH3), blood gas analysis (O2, CO2) are measured using potentiometry.
- Environmental Analysis: Potentiometry is used to analyze ions in environmental samples like water.
- Potentiometric Titrations: It is used to determine equivalence points during titrations.
- Food Processing: Potentiometry is used to measure properties in the food processing industry.
- Detergent Manufacturing: It is used in the detergent manufacturing industry to measure certain properties.
- Agriculture: Potentiometry is used in agriculture for various measurements.
These applications leverage the ability of potentiometry to provide accurate and precise measurements of ionic activity in a solution.
Polarography
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czechoslovak chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959. In its most simple form, polarography can be used to determine concentrations of electroactive species in liquids by measuring their mass-transport limiting currents. It is an electrochemical voltammetric technique that employs a mercury drop as a working electrode. The potential of the working mercury drop electrode is linearly changed in time, and the electrode current is recorded at a certain time just before the mercury drop dislodges from a glass capillary from where the stream of mercury emerges.

Principle
Polarography is an electrochemical method of analysis based on the measurement of current flow resulting from the electrolysis of a solution at a polarizable microelectrode as a function of applied voltage. The principle of polarography is that a gradually increasing voltage is applied between two electrodes, one of which is polarizable (dropping mercury electrode) and the other is non-polarizable. The current flowing between the two electrodes is recorded. A sigmoid shape current-voltage curve is obtained from which half-wave potential as well as diffusion current is calculated. The diffusion current is used for the determination of the concentration of the substance. The half-wave potential is characteristic of every element. The Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the non-polarizable electrode, i.e., the substance reduced or oxidized at the dropping mercury electrode (polarizable electrode).
Ilkovic equation
The Ilkovic equation is a fundamental relation used in polarography that describes the relationship between the diffusion current (id) and the concentration of the substance reduced or oxidized at the dropping mercury electrode. The equation is given by,
Id = 607nD ½ m 2/3 t 1/6 C
Where,
id is the diffusion current in microamperes,
607 is a constant that includes various numerical factors, including the Faraday constant,
n is the number of electrons involved in the electrode reaction,
D is the diffusion coefficient in cm2.sec-1,
m is the weight of Hg flowing via the capillary in mg.sec-1,
t is the drop time in seconds,
C is the concentration in mmol/L.
This equation is used to measure the concentration of substances using diffusion currents in polarography.
Dropping mercury electrode (DME)
The Dropping Mercury Electrode (DME) is a working electrode made of mercury and used in polarography.
Construction
- The DME consists of three components, a capillary, a mercury reservoir vessel, and a standing tube with an adjacent stopcock.
- Mercury is passed through an insulating capillary as a droplet.
- At the end of the capillary, the size of the droplet is increased until it has a diameter of about one millimetre.
- The reservoir vessel contains mercury that is connected to an electrical system through tungsten contact mercury wells.
Working
- The DME is polarizable and can act as an anode as well as a cathode.
- The pool of mercury acts as a counter electrode, i.e., it will act as an anode if DME is the cathode or vice-versa.
- To the analyte solution placed in a glass beaker, supporting electrolyte such as KCl is added.
- Pure nitrogen/hydrogen gas is bubbled through the solution, to take out oxygen.
- Then gradually increasing voltage is applied to the polarographic cell, typically by going to a more negative decomposing potential and current is recorded.
- An inverse graph of voltage versus current is drawn.
- Consequently, Polarograph is this graph and Polarogram is its apparatus.
Rotating platinum electrode (RPE)
The Rotating Platinum Electrode (RPE) is a type of electrode used in electrochemical studies to investigate the kinetics of electrode reactions.
Construction
- The RPE consists of a thin layer of platinum deposited on a glass or quartz rod.
- This rod is attached to a motor to rotate the electrode.
- The electrode is usually immersed in an electrolyte solution.
- It is connected to a potentiostat, which controls the potential applied to the electrode and measures the resulting current.
Working
- The working principle of an RPE is based on the fact that the rate of an electrochemical reaction is proportional to the concentration of the reactants at the electrode surface.
- By rotating the electrode at a constant speed, the concentration of reactants at the electrode surface is constantly refreshed.
- This allows for accurate measurement of the reaction kinetics.
- The RPE is commonly used in a technique called cyclic voltammetry, which involves applying a potential waveform to the electrode and measuring the resulting current.
- By varying the potential waveform, information about the electrochemical reaction mechanism and kinetics can be obtained.
Applications
Polarography has a wide range of applications in various fields.
- Trace Analysis: Polarography is well-suited for detecting and quantifying trace amounts of electroactive species, such as metal ions, organic compounds, and biomolecules.
- Environmental Monitoring: It is used for environmental analysis of air, water, soil, and sea water contaminants.
- Pharmaceutical Analysis: Polarography can be used to analyze drugs and pharmaceuticals. It is particularly useful in determining the concentration of compounds that have electroactive groups, such as neurotransmitters and hormones.
- Food Analysis: Polarography is used to analyze the concentration of various components in food, such as vitamins, amino acids, and trace elements. It can be used to detect adulteration or contamination in food products.
- Biochemical Analysis: Polarography can be used to analyze biological samples such as blood and urine. It is useful in detecting the presence of certain biomolecules such as glucose and cholesterol.
- Electrochemical Kinetics: Polarography is used to study the kinetics of electrochemical reactions.
- Speciation Analysis: Polarography can be used for speciation analysis.
Overall, polarography is a versatile and useful analytical tool that has many applications in various fields, including environmental monitoring, pharmaceuticals, food analysis, and biological research.
Summary
Electrochemical methods are a class of techniques in analytical chemistry that study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be categorized into several types depending on which aspects of the cell are controlled and which are measured. Potentiometry passively measures the potential of a solution between two electrodes, affecting the solution very little in the process. Conductometry measures a property of the solution in the electrochemical cell. Polarography is an electrochemical voltammetric technique that employs a mercury drop as a working electrode. The potential of the working mercury drop electrode is linearly changed in time, and the electrode current is recorded at a certain time just before the mercury drop dislodges from a glass capillary from where the stream of mercury emerges. These methods provide valuable insights into the concentration or reactivity of an analyte.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “Electrochemical Methods”