Enzymes
Enzymes are remarkable biological catalysts that accelerate chemical reactions within living organisms. These molecular workhorses are essential for processes like digestion (breaking down nutrients), energy transformation, and macromolecule synthesis. Typically composed of proteins, enzymes have specialized pockets called active sites where they interact with substrates. Examples include amylase (digests starch), DNA polymerase (involved in DNA replication), and catalase (breaks down hydrogen peroxide). Beyond biology, enzymes find applications in industry (brewing, cheese-making) and medicine. In this article we will study enzyme nomenclature, enzyme kinetics, enzyme inhibitors, regulation of enzymes, coenzymes and therapeutic applications of enzymes.
Properties of enzymes
Catalytic Activity: Enzymes are exceptional catalysts. They initiate and accelerate chemical reactions within living organisms. Without enzymes, many essential reactions would occur too slowly to sustain life.
pH-Specific Activity: Each enzyme has an optimal pH range in which it functions most effectively. Deviating from this range can reduce its activity. For example, stomach enzymes work best in the acidic environment of your stomach.
Specificity: Enzymes are highly specific. Each one recognizes a particular substrate (the molecule it acts upon) due to its unique shape and active site. Think of enzymes as lock-and-key pairs: Only the right key (substrate) fits into the specific lock (enzyme’s active site).
Temperature Sensitivity: Enzymes function optimally within a specific temperature range. Too much heat (or extreme cold) can denature them, altering their shape and rendering them ineffective.
Reversibility: Enzymes can catalyze reactions in both forward and reverse directions. However, they don’t dictate the overall direction of a biochemical pathway; other factors determine that.
Organized Sequences: Enzymes work in organized sequences. They participate in hundreds of stepwise reactions, breaking down nutrients, conserving energy, and building biological macromolecules.
Chemical Nature: Traditionally, enzymes were thought to be exclusively proteins. However, since the 1980s, we’ve discovered ribozymes—catalytic RNAs. They consist of amino acid chains (polypeptides) that determine their unique structure and function.
IUB classification of enzymes
The International Union of Biochemistry (IUB) is a global non-governmental organization dedicated to advancing the fields of biochemistry and molecular biology. The IUB’s primary mission is to promote research and education in biochemistry and molecular biology worldwide. It plays a crucial role, especially in countries where these scientific disciplines are still in their early stages of development. By fostering collaboration, knowledge exchange, and scientific advancement, the IUB contributes to the growth of these fields. For enzyme classification IUB has set some rules as follows.
EC Numbers and Classes
Enzymes are identified by EC (Enzyme Commission) numbers. These unique codes help us understand the type of reaction an enzyme catalyzes and the substrates it uses. The IUB classification system divides enzymes into six functional classes based on their roles:
- Oxidoreductases: These enzymes facilitate reactions involving electron transfer. For example, they can convert AH₂ + B into A + BH₂.
- Transferases: They move functional groups (like methyl or phosphate) from one molecule to another. AX + B becomes BX + A.
- Hydrolases: These enzymes break down compounds by adding water. A-B + H₂O yields AH + BOH.
- Lyases: Lyases cleave or form bonds without water involvement. A=B + X-Y transforms into A-B + X + Y.
- Isomerases: They rearrange atoms within a molecule. For instance, converting A to B.
- Ligases: Ligases join two molecules using energy from ATP. A + B + NTP becomes A-B + NDP + P (or NMP + PP).
And in 2018, a seventh class was added: Translocases. They move molecules across membranes.
Breaking Down the EC Number
The EC number consists of four components separated by full stops:
- Class: The first number identifies the type of reaction. For example, class 1 is for oxidoreductases.
- Subclass: The second number provides information about the compound or group involved. For oxidoreductases, it specifies the type of group in the donor (like CH-OH or CH-NH₂).
- Sub-subclass: The third number further specifies the reaction type. For instance, 1.x.1.- indicates NAD+ or NADP+ as the acceptor.
- Serial number: The fourth component uniquely identifies each enzyme within a sub-subclass.
Example Enzyme Entry
Let’s take an illustrative enzyme entry:
- Accepted name: This is the most commonly used name for the enzyme (usually ending in “-ase”).
- Other names: Additional names, if any.
- Reaction: Describes the specific transformation catalyzed.
- Cofactors: Any helper molecules required.
- Comments: Additional relevant information.
Enzyme Class: Oxidoreductases (EC-1)
Definition: Oxidoreductases catalyze reactions involving electron transfer. They facilitate the transfer of electrons from one molecule (the donor) to another (the acceptor).
Example Enzyme: Lactate Dehydrogenase (LDH)
- Reaction: LDH converts lactate (L-lactate) to pyruvate (pyruvic acid) by transferring electrons.
- Substrate: L-lactate (donor) + NAD⁺ (acceptor) → pyruvate + NADH + H⁺
- Function: LDH is crucial in anaerobic metabolism, such as during intense exercise or in red blood cells.
- Clinical Significance: Elevated LDH levels can indicate tissue damage (e.g., heart attacks, liver disease, or cancer).
Nomenclature of enzymes
Enzymes are remarkable proteins that act as catalysts, speeding up complex biochemical reactions in living organisms. They play crucial roles in processes ranging from digestion to DNA replication. When it comes to naming enzymes, there are a few conventions we follow which are described as follows.
Suffix -ase
Most enzyme names end in “ase.” This suffix helps us recognize that a substance is an enzyme. For example:
- Lactase: Catalyzes the hydrolysis of lactose.
- Glucose oxidase: Facilitates glucose oxidation.
- Urease: Involved in the hydrolysis of urea.
Suffix -in
Some of the earliest studied enzymes have names ending in “in.” Examples include pepsin, chymotrypsin, and trypsin.
Prefix Based on Reaction Type
Enzymes are often named based on the type of reaction they catalyze. Here are a couple of examples:
- Enzyme hydrolase: Catalyzes hydrolysis reactions.
- Enzyme oxidase: Catalyzes oxidation reactions.
Two-Part Naming
The standard naming convention involves two parts:
- Name of the substrate: This tells us which molecule the enzyme acts upon.
- Type of reaction catalyzed: The second part ends with the “ase” suffix.
For instance, consider lactate dehydrogenase:
- “Lactate” is the substrate (it acts on lactate).
- “Dehydrogenase” indicates the type of reaction (involving hydrogen transfer).
EC Numbers
The International Union of Biochemistry and Molecular Biology (IUBMB) has developed a systematic classification system called EC numbers (short for “Enzyme Commission numbers”). Each enzyme is assigned a unique EC number based on its function. These numbers provide a structured way to categorize enzymes:
The EC number format is a sequence of four digits (a.b.c.d):
- a: Class (broad category of enzymatic activity).
- b: Subclass (more specific).
- c: Sub-subclass (even more specific).
- d: Sub-sub-subclass (most specific).
The classification doesn’t consider protein structure or amino acid sequence; it’s purely based on the reaction catalyzed.
Here are the six functional classes of enzymes:
- Hydrolases: Involved in hydrolysis reactions (e.g., breaking down molecules with water).
- Oxidoreductases: Catalyze oxidation-reduction reactions (transfer of electrons).
- Lyases: Facilitate the removal of groups from substrates without hydrolysis or oxidation.
- Transferases: Transfer functional groups between molecules.
- Ligases: Join two molecules together, often using ATP.
- Isomerases: Rearrange atoms within a molecule to form isomers.
Enzyme kinetics
Enzyme kinetics, deals with enzyme reactions which are time-dependent and explains the mechanisms of enzyme catalysis and its regulation.
Let’s understand enzyme kinetics as a function for the concentration of the substrate available for the enzyme.
- Start the experiment with a series of tubes which contains substrate, [S].
- At time (t) zero, add some amount of the enzyme.
- Wait for few minutes
- Then, measure the newly formed product concentration. We can also use spectrophotometer, If product absorbs light
- At a time when the amount of substrate is greater than the amount of enzyme, then, the rate is the initial velocity of Vi.
If we plot Vi as a function of [S], following observations will be made:
- At low [S], the initial velocity,Vi, rises linearly with increasing [S].
- When [S] increases, Vi settle down (rectangular hyperbolais formed).
- The asymptote shows Vmax as the maximum velocity of the reaction.
- The substrate concentration which produces a Vi equal to one-half of Vmax is called the MichaelisMenten constant (Km).
Km is (approximately) inversely related to the maximum reaction velocity, or strength of binding between the enzyme and its substrate. Lower the Km value, higher is the affinity for its substrate.
Michaelis Menten equation
In 1913, Michaelis (1875–1949) and Menten (1879–1960, proceeded the work which was previously done by Frenchchemist V Henri (1872–1940), developed a mechanism to explain how the initial rate of enzyme catalysed reactions depends on the concentration.
Derivation of Michaelis-Menten equation
Few considerable assumptions can be made for the MichaelisMenten equation derivation:
- Assuming that reverse reaction (P→ S) is negligible
- Assuming, there exists only a single central complex (ES). i.e. ES breaks down to E + P.
- Considering a situation when, [S] >> [E].Then the immediate interaction of S and E to form ES does not significantly affect free [S].
- Usually, ([S]-[ES])/[S] ≥ 99.9%
Considering the above assumptions, the reaction scheme is as follows:
Following are two parts for this reaction:
- Formation of ES complex (a second order process)
- The breakdown of ES complex to product P and free enzyme E (a first order process).
And the final Michaelis-Menten equation is as given below :
v = {Vmax[S]} / {[S]+ KM}
Vmax = The maximum velocity achieved by the system, at maximum (saturating) substrate concentrations
KM (the Michaelis constant) = substrate concentration at which the reaction velocity is 50% of the Vmax. Its unit is mM. It is also the concentration at which concentration of substrate is half the maximal velocity is observed.
[S] = concentration of the substrate S.
Lineweaver-Burk Plot (Double Reciprocal Plot)
On the other hand, It is observed that in order to determine the value of Vmax, the plot of V0 vs. [S] is not useful since tracing the value of Vmax under very high substrate concentrations is difficult. So, American chemists H. Line weaver and Burk employed the double-reciprocal plot. These are known as the Lineweaver–Burk plot.
These are also called double-reciprocal plots.
1/V°= (K / V°[S]) + (1 / Vmax)
KM and Vmax is achieved from intercepts and slope of the straight line from graph.
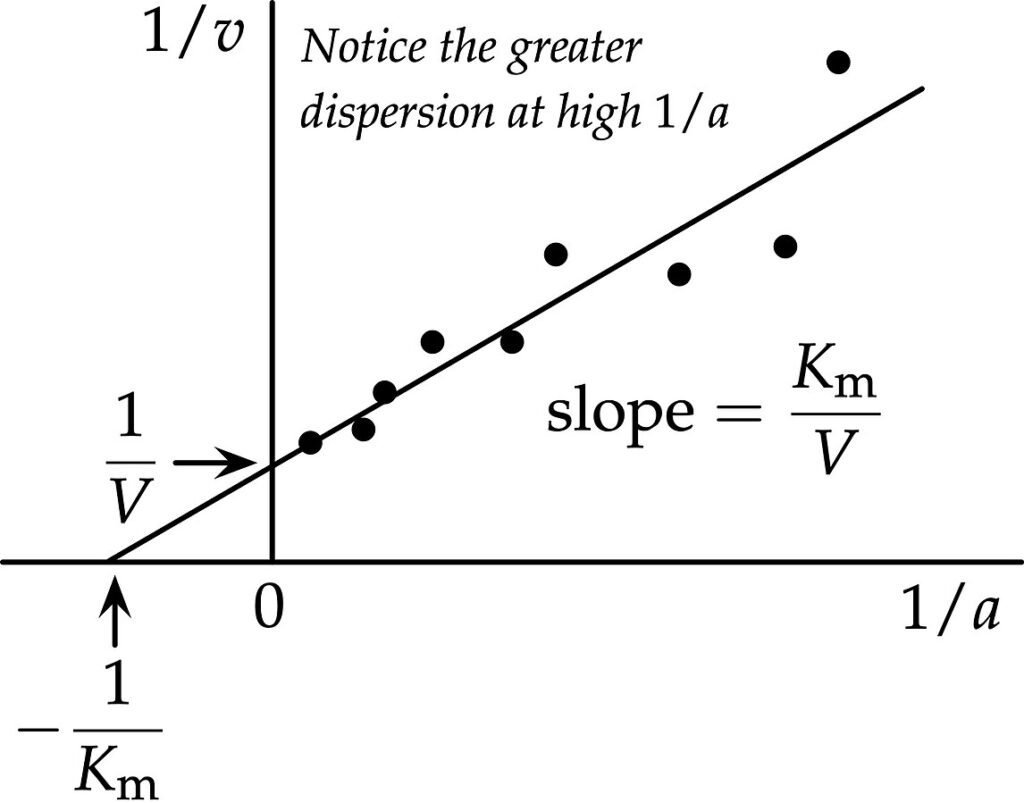
One of the drawbacks of Lineweaver–Burk plot is that it is more susceptible to error. Another disadvantage is that at high substrate concentrations, the plot tends to compresses the data points into a small region and puts more emphasis on the points at lower substrate concentrations, which are often the least accurate.
Enzyme Inhibitors
An enzyme inhibitor is a molecule that binds to an enzyme and interferes with its activity. Enzymes, those remarkable protein catalysts, speed up essential chemical reactions in living organisms. Enzymes typically have a specific active site—a specialized region where they interact with their substrate (the molecule they act upon).
Inhibitors can either:
- Bind to the enzyme’s active site, preventing the substrate from binding (competitive inhibition).
- Bind elsewhere on the enzyme, altering its shape and blocking catalysis (non-competitive or allosteric inhibition).
Types of Enzyme Inhibitors
Let’s explore a few examples:
Competitive Inhibitors
These inhibitors compete with the substrate for the enzyme’s active site.
Examples:
- Statins: Used to lower cholesterol levels by inhibiting HMG-CoA reductase (an enzyme in cholesterol synthesis).
- Methotrexate: Competes with folic acid in inhibiting dihydrofolate reductase (important for DNA synthesis).
Non-competitive Inhibitors (Allosteric Inhibitors)
These inhibitors bind to a different site on the enzyme (not the active site) and alter its shape.
Examples:
- ATP: Allosterically inhibits phosphofructokinase in glycolysis.
- Triclosan: Used in disinfectants; inhibits bacterial enoyl-acyl carrier protein reductase.
- Irreversible Inhibitors:
These form a strong chemical bond with the enzyme, rendering it inactive until the bond is broken.
Example:
Aspirin: Irreversibly inhibits COX enzymes involved in inflammation and pain pathways.
Applications
Enzyme inhibitors have diverse applications:
- Medicine: Many drugs are enzyme inhibitors. For instance:
- Protease inhibitors: Used to treat HIV/AIDS.
- Methotrexate: Used in chemotherapy and rheumatic arthritis treatment.
- Pesticides and Herbicides: Some synthetic inhibitors target specific enzymes in pests or weeds.
- Chemical Warfare Agents: Certain nerve agents inhibit acetylcholinesterase.
- Balance in Cells: Inhibitors help regulate metabolic pathways by controlling enzyme activity.
Regulation of Enzymes
Enzymes are the molecular workhorses that orchestrate biochemical reactions within cells. To maintain metabolic balance and adapt to changing conditions, cells tightly regulate enzyme activity. Here are some key mechanisms:
Enzyme Induction
- Cells can increase the production of specific enzymes in response to environmental cues or substrate availability.
- When a cell encounters a particular substrate or experiences specific conditions, it activates gene expression for relevant enzymes.
- Example: The liver induces cytochrome P450 enzymes when exposed to drugs or foreign compounds. These enzymes help metabolize and detoxify these substances.
Enzyme Repression
- Conversely, cells can decrease enzyme production when the substrate is scarce or when certain products accumulate.
- Repression prevents unnecessary energy expenditure on enzymes that aren’t immediately needed.
- Example: If a cell has sufficient amino acids, it represses the production of enzymes involved in amino acid synthesis.
Allosteric Regulation
Allosteric regulation involves molecules binding to sites other than the enzyme’s active site (allosteric sites). These binding events cause conformational changes in the enzyme, affecting its activity.
Positive Allosteric Regulation
- Activators bind to allosteric sites, enhancing enzyme activity.
- Example: Hemoglobin’s binding of oxygen—an increase in oxygen concentration positively regulates hemoglobin’s affinity for more oxygen.
Negative Allosteric Regulation
- Inhibitors bind to allosteric sites, reducing enzyme activity.
- Example: ATP acts as a negative allosteric regulator for phosphofructokinase (an enzyme in glycolysis). High ATP levels signal that the cell has sufficient energy, so glycolysis is inhibited.
Allosteric regulation allows cells to fine-tune metabolic pathways dynamically.
Feedback Inhibition
Key metabolic enzymes are often inhibited by the end product of the pathway they control.
How does this work?
- Imagine an assembly line where each worker produces a specific product. As the product accumulates, it signals the workers to slow down or stop.
- Similarly, feedback inhibition prevents excessive production of a metabolite.
- Example: In amino acid biosynthesis, the final amino acid in the pathway inhibits the enzyme responsible for its own synthesis.
Compartmentalization
- Cells store enzymes in specific compartments (organelles) to prevent damage or create optimal conditions.
- Example: Lysosomes contain enzymes that break down cellular waste. Keeping them isolated prevents accidental digestion of essential components.
Therapeutic Applications of Enzymes and Isoenzymes
Replacing Deficient Enzymes
Some individuals have genetic deficiencies that result in insufficient enzyme production. Therapeutically, we can provide these missing enzymes to restore normal metabolic processes.
Examples:
- Enzyme Replacement Therapy (ERT) for lysosomal storage disorders (e.g., Gaucher disease, Fabry disease, Pompe disease). Patients receive intravenous infusions of the missing enzyme (e.g., glucocerebrosidase, alpha-galactosidase, acid alpha-glucosidase).
- Pancreatic Enzyme Replacement: Used in pancreatic insufficiency (e.g., due to chronic pancreatitis or cystic fibrosis) to aid digestion by replacing deficient pancreatic enzymes (lipase, amylase, protease).
Dissolving Clots (Thrombolytic Therapy)
Enzymes can break down blood clots (thrombi) and restore blood flow.
Example:
Tissue Plasminogen Activator (tPA): Used in acute ischemic stroke and myocardial infarction (heart attack) to dissolve blood clots.
Targeting Cancer Cells
Enzymes can selectively target cancer cells or enhance the effects of chemotherapy.
Example:
Asparaginase: Depletes asparagine (essential for cancer cell growth) in acute lymphoblastic leukemia (ALL) treatment.
Reducing Oxidative Stress
Antioxidant enzymes combat oxidative damage caused by free radicals.
Example:
Superoxide Dismutase (SOD): Converts superoxide radicals into less harmful molecules.
Diagnostic Applications of Enzymes and Isoenzymes
Biomarkers for Disease Diagnosis
Different tissues express specific sets of isoenzymes. Measuring these can aid in diagnosing various conditions.
Examples:
- Cardiac Troponin I and CK-MB: Used to diagnose myocardial infarction (heart attack).
- Alkaline Phosphatase (ALP) and Gamma-Glutamyl Transferase (GGT): Indicators of liver and bone diseases.
Prognosis and Monitoring
Isoenzymes help predict disease outcomes and monitor therapy response.
Examples:
- Lactate Dehydrogenase (LDH) Isoenzymes: Predict testicular cancer outcome.
- Alpha-Fetoprotein (AFP) to Des-Gamma-Carboxy Prothrombin (DCP) Isoenzyme Ratio: Predicts hepatocellular carcinoma recurrence.
- Prostate-Specific Antigen (PSA): Monitors prostate cancer therapy response.
- Thyroglobulin (Tg): Monitors thyroid cancer recurrence.
Targeted Therapy
Selective inhibitors of specific isoenzymes can be used for targeted effects.
Example:
COX-2 Inhibitors: Treat inflammation and pain while minimizing side effects compared to non-specific COX inhibitors.
Coenzymes: Structure and Biological Functions
Definition: A coenzyme is an organic, non-protein compound that binds to an enzyme, working hand-in-hand to catalyze specific reactions. Unlike enzymes, which are proteins, coenzymes are smaller molecules that play essential roles in various metabolic pathways. coenzymes are like the backstage crew—essential for the show to go on smoothly.
Functions of Coenzymes
- Enzyme Activation: Coenzymes activate enzymes, transforming them from inactive forms (apoenzymes) to active forms (holoenzymes). Without coenzymes, enzymes wouldn’t function effectively.
- Carrier Molecules: Coenzymes often act as carriers, shuttling specific atoms or functional groups between molecules during reactions.
- Metabolic Pathways: Coenzymes participate in critical metabolic processes, ensuring energy production, biosynthesis, and cellular maintenance.
Structure and Types
Coenzymes come in various forms:
Organic Coenzymes
These contain carbon and are often derived from vitamins. They loosely bind to an enzyme’s active site to facilitate reactions.
Examples:
- Nicotinamide Adenine Dinucleotide (NAD): Derived from vitamin B3 (niacin), NAD plays a central role in redox reactions, shuttling electrons during cellular respiration.
- Coenzyme A (CoA): Derived from pantothenic acid (another B vitamin), CoA participates in fatty acid metabolism and the citric acid cycle.
Inorganic Coenzymes (Cofactors)
These lack carbon and are typically metal ions. They also bind to an enzyme’s active site.
Examples:
- Iron (Fe): Involved in electron transport chains.
- Zinc (Zn): Required for DNA synthesis and various enzymatic reactions.
- Copper (Cu): Essential for iron metabolism and antioxidant defense.
Prosthetic Groups
These tightly or covalently bind to enzymes.
Examples:
- Heme: A prosthetic group in hemoglobin and myoglobin, allowing them to carry oxygen.
- Biotin: A covalently attached prosthetic group in enzymes involved in carboxylation reactions.
Importance and Sources
Most organisms cannot synthesize coenzymes in sufficient quantities, so they must obtain them from external sources:
- Vitamins: Many coenzymes are derived from vitamins.
- Water-soluble vitamins (such as B complex vitamins and vitamin C) contribute to coenzyme production.
- For instance, vitamin B3 (niacin) is a precursor for NAD.
- Dietary Intake: Consuming a balanced diet ensures an adequate supply of coenzymes.
Summary
In summary, enzymes and their isoenzymes are vital players in both diagnosis and treatment. As we continue to develop more precise methods for detecting and targeting them, personalized medicine will advance further.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

2 thoughts on “Enzymes”