HLB scale, solubilization and detergency
The Hydrophilic-Lipophilic Balance (HLB) scale is essential in formulating effective pharmaceutical and cosmetic products. It measures the balance between the water-loving and oil-loving parts of a surfactant molecule, influencing solubilization and detergency. Solubilization enhances the solubility of poorly soluble compounds, improving drug efficacy and bioavailability. Detergency, on the other hand, involves removing dirt and oil from surfaces, with the HLB value determining the effectiveness of emulsifying oils and suspending dirt particles. Mastering the HLB scale allows formulators to create safe, effective, and user-friendly products across various industries.
HLB scale
The Hydrophilic-Lipophilic Balance (HLB) scale is a fundamental concept in the formulation of surfactants and emulsifiers, particularly in the pharmaceutical and cosmetic industries. Here’s a detailed look at its formation history and uses in pharmacy:
Formation History
The HLB scale was developed by William C. Griffin in 1949 to quantify the balance between the hydrophilic (water-attracting) and lipophilic (oil-attracting) properties of surfactant molecules. Griffin’s method assigns a numerical value to a surfactant based on its molecular structure, indicating the degree of hydrophilicity or lipophilicity. The scale typically ranges from 0 to 20, where lower values indicate more lipophilic (oil-loving) surfactants, and higher values indicate more hydrophilic (water-loving) surfactants.
In 1957, Davies proposed an alternative method for calculating HLB values, which takes into account the effect of different chemical groups within the molecule. This method provides a more nuanced understanding of the surfactant’s properties by considering the strength of various hydrophilic and lipophilic groups.
Principles of the HLB Scale
- Definition: The HLB scale quantifies the degree to which a surfactant molecule possesses hydrophilic or lipophilic characteristics.
- Hydrophilic and Lipophilic Groups: Surfactant molecules typically consist of a hydrophilic head and a lipophilic tail. The hydrophilic group interacts with water, while the lipophilic group interacts with oil or other hydrophobic phases.
- HLB Range: The HLB scale ranges from 0 to 20. Low HLB values (1-6) indicate lipophilic surfactants, intermediate values (7-13) suggest surfactants with both hydrophilic and lipophilic characteristics, and high values (14-20) represent hydrophilic surfactants.
Calculation of HLB
Griffin’s Method: For non-ionic surfactants, the HLB value is calculated using the formula:
HLB= (20Mh)/M
where ( M_h ) is the molecular mass of the hydrophilic portion, and ( M ) is the molecular mass of the whole molecule.
Davies’ Method: This method calculates the HLB value based on the number and type of hydrophilic and lipophilic groups in the molecule, providing a more detailed assessment.
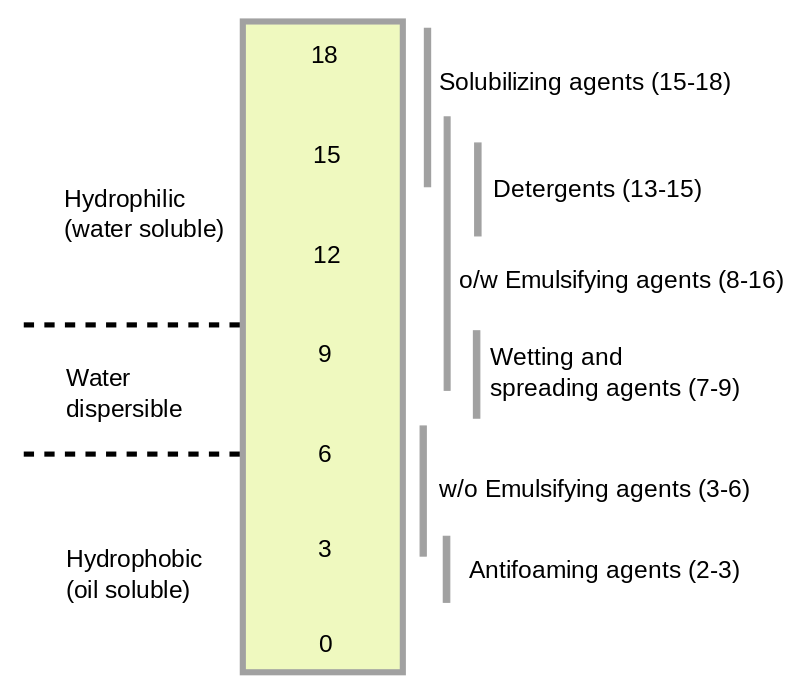
Applications
- Emulsion Stability: The HLB value is crucial in formulating stable emulsions. Surfactants with appropriate HLB values can stabilize oil-in-water (O/W) or water-in-oil (W/O) emulsions, which are essential in various pharmaceutical formulations.
- Drug Solubility: By selecting surfactants with suitable HLB values, formulators can enhance the solubility of poorly soluble drugs, improving their bioavailability and therapeutic efficacy.
- Drug Delivery Systems: The HLB concept aids in designing drug delivery systems such as microemulsions, liposomes, and nanoparticles, ensuring the stability and effectiveness of these systems.
- Cosmetics and Personal Care: In the formulation of creams, lotions, and other personal care products, the HLB scale helps in selecting surfactants that provide the desired texture and stability.
- Food and Agriculture: Beyond pharmaceuticals, the HLB scale is also used in the food industry for selecting emulsifiers that enhance the stability and texture of food products, and in agriculture for formulating agrochemicals and crop protection products.
Solubilization
Solubilization is the process by which a solute (the substance being dissolved) becomes uniformly dispersed in a solvent (the substance doing the dissolving), forming a stable, homogeneous solution. This process is crucial in various fields, including pharmaceuticals, chemistry, and industrial applications.
Mechanisms of Solubilization
- Micellar Solubilization: Surfactants form micelles in a solution, with hydrophilic exteriors and hydrophobic interiors, allowing them to solubilize hydrophobic substances within the micellar core.
- Cosolvency: The use of co-solvents can increase a solute’s solubility by altering the interactions between solute molecules and the solvent.
- Complexation: Solubilization can occur when a solute forms a complex with another substance, such as cyclodextrins or metal ions, improving solubility.
- Particle Size Reduction: Techniques like milling or micronization reduce the particle size of a solute, increasing its surface area and aiding in solubilization.
Factors Influencing Solubilization
- Nature of Solute and Solvent: The chemical nature and polarity of the solute and solvent significantly affect solubilization.
- Temperature: Generally, solubility increases with temperature, although this is not a universal rule.
- pH: The pH of a solution can influence the solubility of substances, especially those with acidic or basic functional groups.
- Surfactant Concentration: In micellar solubilization, the concentration of surfactants affects micelle formation and stability.
- Co-solvent Selection: The choice of co-solvent can significantly impact solubility, with appropriate polarity and interaction with the solute enhancing solubilization.
Applications of Solubilization
- Pharmaceuticals: Enhancing the solubility of poorly soluble drugs to improve bioavailability and therapeutic effects.
- Food and Beverage Industry: Producing emulsions, flavor solutions, and nutrient delivery systems.
- Cleaning Products: Formulating cleaning products with surfactants and solubilizing agents to enhance the solubility of greases and oils.
- Cosmetics: Solubilizing fragrance oils, vitamins, and other active ingredients in cosmetic formulations.
Detergency
Detergency refers to the ability or power of a substance to clean or remove dirt, stains, and impurities from surfaces. This property is primarily associated with detergents, which are substances designed to break down and remove grease, oil, and dirt through a combination of chemical and physical actions.
Mechanisms of Detergency
- Surfactant Action: Detergents contain surfactants, which reduce the surface tension of water, allowing it to spread and wet surfaces more effectively. Surfactants have hydrophilic (water-attracting) and lipophilic (oil-attracting) ends, which help emulsify oils and suspend dirt particles in water.
- Emulsification: The surfactants in detergents form micelles around oil and grease particles, breaking them into smaller droplets that can be easily washed away.
- Suspension: Detergents keep dirt and oil particles suspended in the cleaning solution, preventing them from redepositing on the cleaned surface.
Factors Influencing Detergency
- Water Hardness: Hard water contains high levels of calcium and magnesium ions, which can reduce the effectiveness of detergents by forming insoluble salts. Modern detergents often include water softeners to counteract this effect.
- Temperature: Higher temperatures generally enhance the cleaning power of detergents by increasing the solubility of oils and the activity of surfactants.
- pH Levels: The pH of the cleaning solution can affect the performance of detergents. Alkaline conditions often improve the removal of organic soils and greases.
- Mechanical Action: Agitation or scrubbing helps to physically dislodge dirt and enhance the action of detergents.
Applications of Detergency
- Household Cleaning: Detergents are widely used in household cleaning products, including laundry detergents, dishwashing liquids, and surface cleaners.
- Industrial Cleaning: In industrial settings, detergents are used to clean machinery, equipment, and workspaces, ensuring hygiene and operational efficiency.
- Pharmaceuticals: In the pharmaceutical industry, detergents are used to clean equipment and surfaces, ensuring that they are free from contaminants and residues.
- Personal Care: Detergents are key ingredients in personal care products like shampoos, body washes, and facial cleansers, where they help to remove oils, dirt, and impurities from the skin and hair.
Summary
The Hydrophilic-Lipophilic Balance (HLB) scale, developed by Griffin, quantifies the balance between the hydrophilic (water-loving) and lipophilic (oil-loving) portions of surfactant molecules. This scale, ranging from 1 to 40, helps predict the solubility and emulsifying properties of surfactants. Surfactants with low HLB values are more oil-soluble, while those with high HLB values are more water-soluble. Solubilization involves creating a stable isotropic solution of an otherwise insoluble substance, often using surfactants. Detergency refers to the ability of surfactants to remove dirt and oil by reducing surface tension and forming micelles.
For practice MCQ on this article, click here.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “HLB scale, solubilization and detergency”