Impurities
Impurities in pharmaceutical substances are the unwanted chemical substances in the active pharmaceutical ingredient (API) or developed during formulation or upon ageing of both API and formulation. In simple words, any component that is not a formulation ingredient is known as impurity. The presence of these impurities affects the safety and efficacy of the pharmaceutical product. So, in pharmaceutical industry, it is important to use pure substances so that they can be safely used. However, it is impossible to get absolutely pure chemical substance. The control of impurities is a critical issue as impurities can develop at any stage of manufacturing and purification.
Sources of impurities
Impurities can originate in the drug from various sources and phases of synthesis and manufacturing of pharmaceutical product. Based on their sources, impurities can be classified as follows
Sources of organic impurities
- Impurities originating from API synthesis
- Starting materials and intermediates
- Reagents, ligands and catalysts
- By products of the synthesis
- Products of over reaction
- Products of side reactions
- Degradation of the API or other excipients
Enantiomeric impurities
Most of the drugs used in pharmaceutical industry are natural products having enantiomeric properties optical isomers). Enantiomers are the two stereoisomers that are non-superimposable mirror images of each other. In some drugs only one enantiomer shows therapeutic action, hence other one becomes impurity.
Types of impurities
According to International Council for Harmonisation (ICH) there are three types of impurities present in pharmaceutical substances, i.e. organic impurities, inorganic impurities, residual impurities.
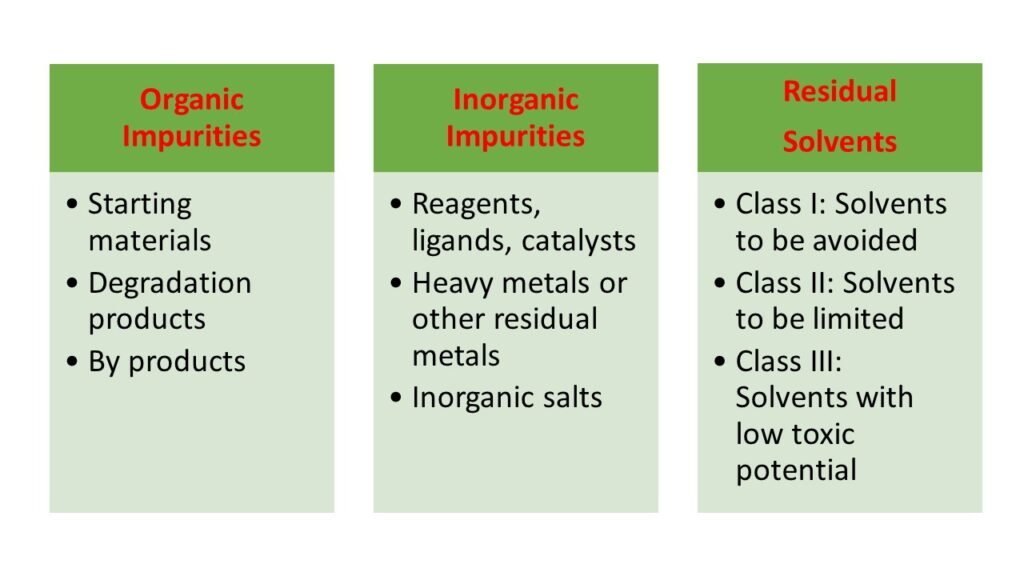
Organic impurities
These are the process related pharmaceutical impurities. Organic impurities mostly arise due to synthesis, purification and storage of the drug substances and excipients used in the manufacturing. For example, by products, intermediates, degradation products, ligands and catalysts.
Inorganic impurities
Inorganic impurities also arise in the manufacturing process. These impurities can be detected and quantified using pharmacopeial standards. Some examples are reagents, ligands, heavy metals, inorganic salts, filter aids and charcoal, etc.
Residual solvents
Residual solvents are used in the manufacturing process can be a source of impurity. Based on their toxicity, these residual solvents are classified in three classes.
- Class I residual solvents: Class I residual solvents are carcinogenic and environmentally hazardous in nature. They should always be avoided. For example, carbon tetrachloride, 1,2 dichloroethane.
- Class II residual solvents: These solvents contain some level of harmful toxicity and can be used as limited solvents in pharmaceutical preparations. For example, acetonitrile, chloroform, toluene.
- Class III residual solvents: These solvents have negligible toxic potential to humans and can be used widely in pharmaceutical preparations. For example, acetone, anisole, formic acid.
Effects of impurities
As we have discussed, it is impossible to get almost pure substance, some amount of impurity is always present in chemical substance. The impurities present may have some adverse effects.
- Toxic impurities can be injurious
- When impurities are present in traces, they show cumulative toxicity after a certain period of time.
- When impurity is harmless but present in large proportion, can reduce the desired therapeutic effect of drug substance.
- Impurities may bring some physical and chemical changes in chemical substance, reducing their shelf life.
- Some impurities may be incompatible with the other substances.
- Few harmless impurities may change colour, odour, taste of the chemical substance.
Tests for purity
The pharmacopeial committee provides guidelines for the identification of impurity and their permissible limits. Some impurities are harmful but can be tolerated by body, some are harmless and they do not interfere in action of drug substance. Various pharmacopoeias prescribe ‘tests for purity’, for substances use in pharmaceutical manufacturing. These tests detect the impurities in chemical substances. Pharmacopoeia also gives the limit of tolerance for these impurities.
Colour, odour and taste
Impurities can be identified based upon their morphological characteristics (colour, odour, taste).
Physico-chemical constants
Physico-chemical properties like melting point, boiling point, optical rotation, refractive index (RI), acid value, iodine value, saponification value can be used to detect the impurities. When impurity is present in very low concentration, these properties fail to detect them.
Acidity, alkalinity and pH
Chemical substances prepared from reactions of acids or alkalis may generate some sort of impurity. Solutions of certain substances have specific pH at a given concentration. Hence presence of impurity may bring change in pH.
Anions and Cations
The presence of chloride and sulphate ions in pharmaceutical substances are the common impurities. These can be detected using their limit tests.
Insoluble residue
API is clearly soluble in given solvent. If it is contaminated by any impurity the solution becomes turbid or cloudy. Upon filtration and weighing one can measure the impurity present in chemical substance.
Ash, water insoluble ash
Ash value of crude drugs is used to determine the water insoluble impurity such as heavy metals or minerals.
Conclusion
Impurity is the any unwanted substance, which is not a part of drug formulation, present in API or formulation. Impurity can arise due to any one of the manufacturing process, storage, degradation, excipients or contaminants. It is almost impossible to obtain 100 percent pure substances in the manufacturing of API or formulations, hence pharmacopoeia sets some limits for the detection and quantification of impurities.
Frequently asked questions
What are called impurities?
Any unwanted substance other than the API or excipients used in the formulation is called as impurity.
What is meant by impurities in drugs?
Impurity is any unwanted substance which is not meant to be part of the drug or API.
What is impurity and its sources?
Any unwanted substance other than the API or excipients used in the formulation is called as impurity. According to international Council for Harmonisation (ICH) there are three types of impurities present in pharmaceutical substances, i.e. organic impurities, inorganic impurities, residual impurities.
What is the importance of impurities?
Presence of impurity even in trace amount may affect the efficacy and safety of drug and its formulations. Hence it is important to study impurities and various tests to detect them.
Whether impurities are permissible as per pharmacopoeia?
Yes. Some impurities are permitted up to certain limits as per pharmacopoeia.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

6 thoughts on “Impurities- It’s sources, Types and Test of Purity.”