Photolytic degradation and its prevention
Photolytic degradation is a significant challenge in the pharmaceutical industry, as it can lead to the loss of potency, the formation of toxic byproducts, and a decrease in product quality. This phenomenon occurs when drugs are exposed to light, particularly ultraviolet (UV) radiation. Understanding the mechanisms of photolytic degradation and implementing effective stabilization strategies is crucial to ensure the safety and efficacy of pharmaceutical products.
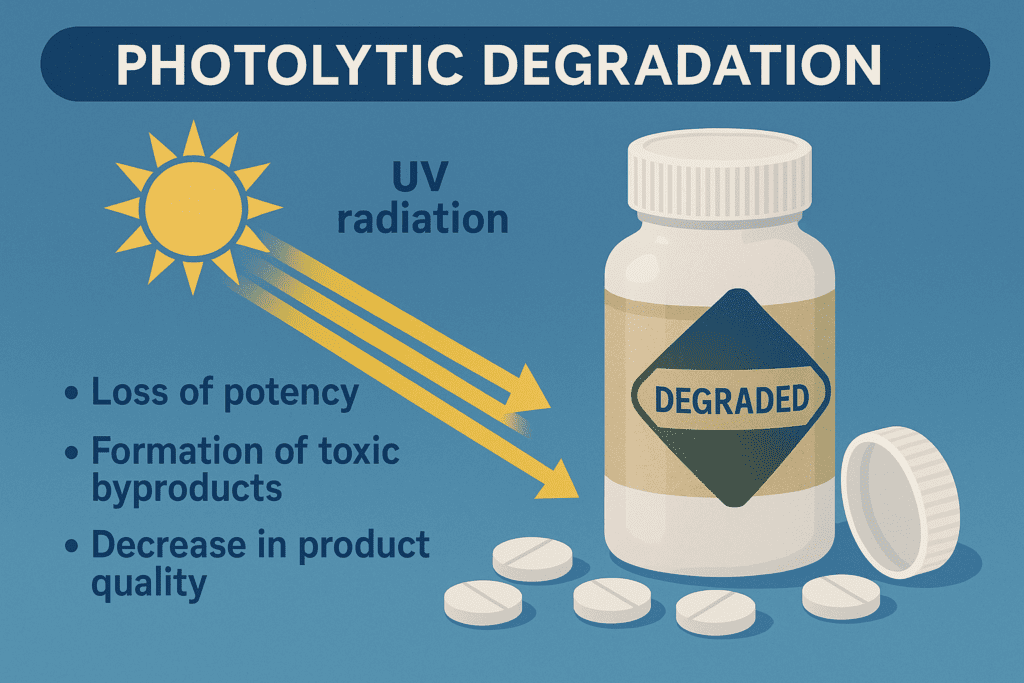
Mechanisms of Photolytic Degradation
Photolytic degradation can occur through various mechanisms, including:
- Direct Photolysis:
- A drug molecule absorbs a photon of light, leading to electronic excitation.
- The excited molecule can undergo bond cleavage, isomerization, or other chemical reactions.
- Indirect Photolysis:
- A photosensitizer molecule absorbs light and generates reactive oxygen species (ROS), such as singlet oxygen and hydroxyl radicals.
- These ROS can react with the drug molecule, leading to its degradation.
Factors Affecting Photolytic Degradation
Several factors influence the rate of photolytic degradation:
- Wavelength of Light: Shorter wavelengths, particularly in the UV range, are more energetic and can induce photochemical reactions.
- Light Intensity: The intensity of light exposure directly affects the rate of photodegradation.
- Oxygen: Oxygen can act as a photosensitizer, accelerating photooxidation reactions.
- Temperature: Higher temperatures can increase the rate of photochemical reactions.
- Drug Structure: The chemical structure of the drug molecule determines its susceptibility to photodegradation.
- Excipients: Excipients can act as photosensitizers or photostabilizers, influencing the rate of photodegradation.
- Packaging Material: The type of packaging material used can affect the amount of light reaching the drug product.
Prevention of Photolytic Degradation
To minimize photolytic degradation, pharmaceutical scientists employ a variety of strategies:
- Formulation Design
- Excipient Selection: Using excipients with photoprotective properties can help shield the drug from light.
- pH Adjustment: Adjusting the pH of the formulation can reduce the rate of photodegradation.
- Complexation: Complexing the drug with suitable ligands can decrease its photosensitivity.
- Packaging
- Light-Resistant Packaging: Using amber glass or opaque plastic containers can block UV radiation.
- Aluminum Foil: Aluminum foil is an effective barrier to light and can be used in blister packs or other packaging formats.
- Light-Stable Blisters: Blister packs with light-stable polymers can provide additional protection.
- Storage Conditions
- Controlled Light Exposure: Storing drugs in dark, cool places can minimize photodegradation.
- Avoid Direct Sunlight: Direct sunlight should be avoided during storage and transportation.
- Chemical Stabilization
- Antioxidants: Adding antioxidants can scavenge free radicals generated during photooxidation.
- Chelating Agents: Chelating agents can bind metal ions that can catalyze photodegradation.
- Photosensitizer Removal: Removing photosensitizers from the formulation can reduce the rate of photodegradation.
Regulatory Considerations
Regulatory agencies, such as the FDA and EMA, have specific guidelines for evaluating the photostability of drug products. These guidelines require manufacturers to conduct photostability studies to assess the impact of light on drug quality and stability.
Conclusion
Photolytic degradation is a significant challenge in the pharmaceutical industry, but it can be effectively mitigated through careful formulation design, appropriate packaging, and proper storage conditions. By understanding the mechanisms of photodegradation and implementing appropriate stabilization strategies, pharmaceutical scientists can ensure the quality, safety, and efficacy of drug products.
For practice MCQ on this article, click here.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us







One response to “Photolytic degradation and its prevention”
[…] Photolytic Degradation And Its Prevention » PHARMACAREERS […]