Solubility of drugs
The word soluble obtains from Latin word solvere meaning to dissolve. Solubility of drugs is defined as the amount of solute dissolved in a specific amount of solvent at specific temperature. Solubility is a physicochemical property of a substance to fully dissolve in another substance. Solubility is generally express in gm/lit. Solubility of drugs or any other substance in a solvent can be expressed quantitatively in numerous terms like percent by mass, percent by volume, molality, molarity, mole fraction and parts per million (ppm).
General terms of solubility
| Term | Parts of solvent per 1 part of solute |
| Very soluble | less than 1 part |
| Freely soluble | 1 – 10 |
| Soluble | 10 – 30 |
| Sparingly soluble | 30 – 100 |
| Slightly soluble | 100 – 1000 |
| Very insoluble | 1000 – 10000 |
| Insoluble/ practically insoluble | More than 10000 |
Saturated solution: In this solution, dissolved solute is in equilibrium phase with the undissolved solute of solid phase.
Unsaturated solution: In this solution, the dissolved solute is in less concentration than saturated solution.
Supersaturated solution: In this solution, the dissolved solute is in more concentration than saturated solution.
Molarity and Molality: The molarity of a solution is the number of moles (gram molecular weight) of solute in 1 liter of solution. The molality is the number of moles of solute in 1 kg of solvent.
Milliequivalents: The unit milliequivalent (mEq) is commonly used clinically in expressing the concentration of an ion in solution.
Mechanism of solute solvent interaction (solubility)
A solute dissolve in solvent when there is change of free energy of both solute and solvent, which depends on several factors.
Dissolution occurs when solute – solvent interaction is similar with solvent – solvent interaction, signified as ‘like dissolves like’. Therefore, polar solute dissolves in polar solvent and non-polar solute dissolves in non-polar solvent. Dissimilarity in nature makes solute insoluble in solvent. Substances dissolve when solvent solute attraction is greater than solvent-solvent attraction and solute-solute attraction. Dissolution stages are,
- Removal of solute molecule from bulk solute molecule
- Formation of cavity in solvent molecule
- Insertion of solute molecule in solvent cavity
Ideal solubility parameters (δ1)
The solubility parameter is a measure of intermolecular forces within the solvent and gives us information on the ability of a liquid to act as a solvent.

Solvation
Solvation is the process of rearranging solvent and solute molecule into solvation complexes, to distribute solute molecules evenly within the solvent. A complex formed of molecule or ion of solute in a solvent is known as a solvation complex. Solvation process is affected by hydrogen bonding and van der Waals forces. Solution of a solute by water is called as hydration.
Association
It is a chemical reaction wherein ions of opposite electrical charge come together in solution to form a distinct chemical entity. Ion’s associate can be classified on the basis of number of ions associated, as ion pairs, ion triplets etc. ions pair classified as contact, solvent shared or solvent separated, fully solvated. Ion’s associate characterize by means of vibrational spectroscopy. Ion pairs are formed when a cation and anion come together. The order of ion closeness in ion pairs is, fully solvated >Solvent-shared > Contact.
Quantitative approach to the factors influencing Solubility of drugs
The temperature has very much significance on the solubility. In endothermic process solubility increases with increase in temperature and vice versa. In exothermic process solubility decrease with decrease in temperature.
Solubility curves: A curve drawn between solubility and temperature is called solubility curve. It indicates the effect of temperature on solubility of substances. There are two types of solubility curves, continuous solubility curve and discontinuous solubility curve.
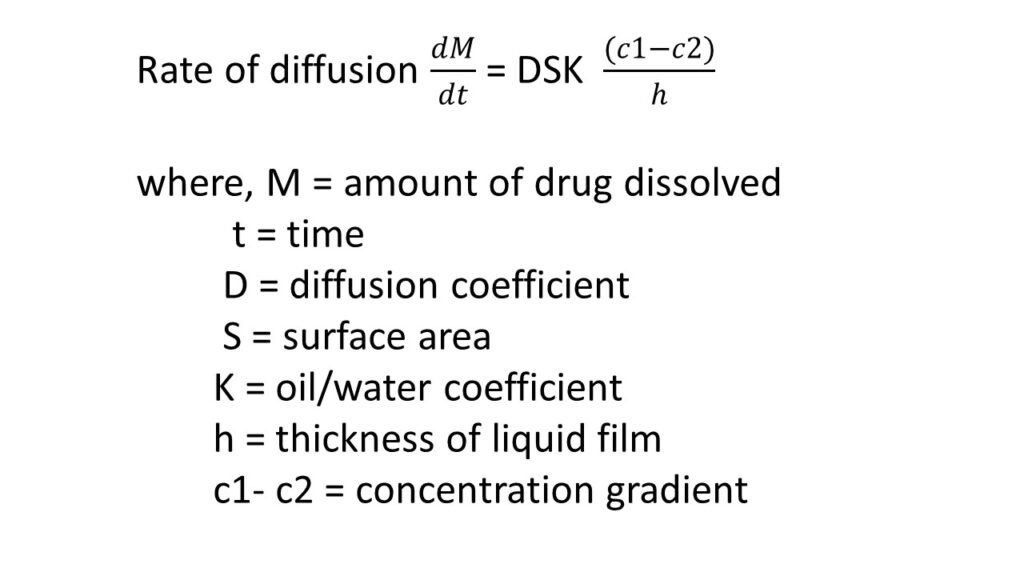
Diffusion principles in biological systems
Diffusion is the movement of matter from higher concentration to lower concentration. The rate of diffusion is depending on temperature, size of particle and size of the concentration gradient.
Solubility of gas in liquids
It is the concentration of dissolved gas in the liquid when it is in equilibrium with the pure gas above the solution. Solubility of gas in liquids is depends on pressure, temperature, chemical reaction, micellar solubilization. At higher gas pressure more gas dissolved in liquids.
Henry’s law
In dilute solution the mass of gas which dissolves in each volume of liquid solvent at constant temperature is directly proportional to partial pressure of gas.
Sg = KHPg
Where, Sg = solubility of gas (mol/L)
KH = Henry law constant
Pg = partial pressure(mmHg)
Solubility of liquids in liquids
Binary solutions
When two or more liquids mixed together, they can be completely miscible, partially miscible or practically immiscible. Completely miscible liquids are uniform in all proportions. Partially miscible liquids form two immiscible liquid layers. Such liquid pair called as conjugated liquid pair. This liquid pairs are temperature specific. The mutual solubility of two conjugated liquid phase increase with increase in temperature called as conjugated temperature. Above this temperature they are soluble in any proportions.
Ideal solutions
It is a homogeneous solution where the interaction between molecules of components is exactly the same to the interactions between the molecules of each component itself. These types of solutions follow the Raoult’s law. If we take two substances A and B they will show, A and A, B and B, A and B intermolecular forces of attraction.
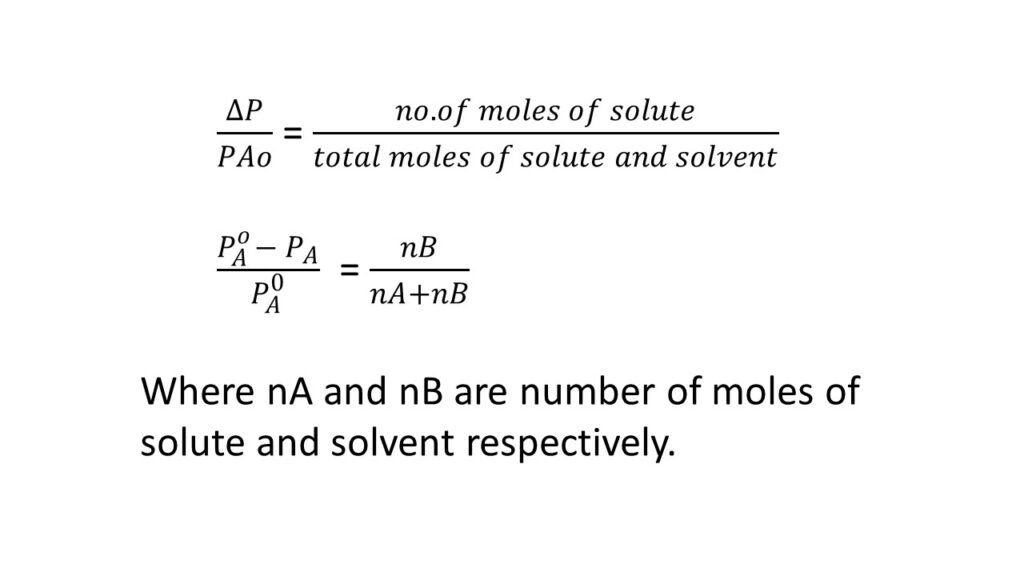
Raoult’s law states that the relative lowering of vapor pressure of a solvent by dissolving a non-volatile electrolyte is equal to the mole fraction of the solute.
Real solutions
When concentration of solute is high, intermolecular forces of solute-solute and solute-solvent are predominant. They deviate from the Roult’s law. Real solutions show change in the total volume of solution when different components mix together.
Colligative property
It is a property of solution that is depended on the ratio between the total number solute particles to the total number of solvent particles. Types are,
- Boiling point elevation
- Freezing point depression
- Relative lowering of vapor pressure
- Osmotic pressure
Critical solution temperature
The temperature at which complete miscibility is reached as the temperature is raised or in some cases lowered, for the two partially miscible liquids in ordinary conditions is called as critical solution temperature, also called as consolute temperature.
Applications
- To determine water content in substances.
- CST is taken as a criterion for the purity of substance.
Distribution law or Nernst’s distribution law
When an excess amount of solute is added to two immiscible liquid phases, it distributes itself between these two phases until saturation. If insufficient amount of solute is added it distributes in a definite ratio, known as partition coefficient. Partition coefficient is a measure of drugs lipophilicity. Partition coefficient is sometimes called distribution coefficient.

Applications
- Partition coefficient helps in selection of type of solvent for a drug.
- Partition coefficient is the best predictor of absorption rate, pKa and solubility of a drug.
- It helps in extraction of drugs from mixtures such as blood and crude plant extracts.
Limitations
- If the solute is present in high concentration, then distribution law does not hold good.
- Samples can be analyzed only if equilibrium is achieved.
- The selected liquid pair must be immiscible with each other.
Frequently asked questions
What is an ideal solution?
It is a homogeneous solution where the interaction between molecules of components is exactly the same to the interactions between the molecules of each component itself. These types of solutions follow the Raoult’s law.
What is solvation?
Solvation is the process of rearranging solvent and solute molecule into solvation complexes, to distribute solute molecules evenly within the solvent.
What are four colligative properties?
- Boiling point elevation
- Freezing point depression
- Relative lowering of vapor pressure
- Osmotic pressure
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us
