Substituents of benzene
Substituents of Benzene, when we talk about substituents in the context of benzene, we’re referring to atoms or groups of atoms that replace one or more hydrogen atoms on the benzene ring. These substituents can significantly influence the reactivity and properties of the benzene compound. Substituents are atoms or groups of atoms that replace one or more hydrogen atoms on a benzene ring. These substitutions give rise to a vast array of compounds with diverse properties. In this article we will see substituents of benzene, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction and some important derivatives of benzene.
Activated vs. Deactivated Rings
- Activated Rings: These are benzene rings with substituents that donate electrons. Examples of activating groups (in order of increasing activation) include amino groups (-NH₂, -NR₂), hydroxyl groups (-OH, -OR), and acylamino groups (-NHCOR).
- Deactivated Rings: These are benzene rings with substituents that withdraw electrons. Examples of deactivating groups (in order of increasing deactivation) include nitro groups (-NO₂), trifluoromethyl groups (-CF₃), carbonyl groups (-COR), cyano groups (-CN), and sulfonic acid groups (-SO₃H).
Reactivity and Electronegativity
- The reactivity of substituted benzene rings depends on the electronegativity of the substituent.
- The most electronegative halogen-substituted benzene ring is the most reactive (least deactivating), while the least electronegative halogen-substituted ring is the least reactive (most deactivating).
- Larger halogens decrease the reactivity of the benzene ring they are attached to.
Reaction Direction
- Activating groups direct electrophilic substitution reactions to the ortho (2-position) or para (4-position) positions. These groups make the ring more nucleophilic.
- Deactivating groups direct the reaction to the meta (3-position) position. However, halogens (which are deactivating) can still direct ortho or para substitution due to their unique behavior.
Resonance and Inductive Effects
- Resonance Effect: This occurs when there is conjugation between the benzene ring and the substituent. It involves the delocalization of π electrons between the ring and the substituent.
- Inductive Effect: Here, the sigma (single bond) electrons are withdrawn from the ring toward the substituent due to the substituent’s higher electronegativity.
Nomenclature
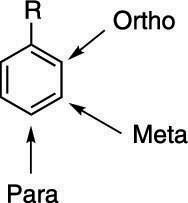
Instead of using numerical positions, we can use the terms ortho (o-), meta (m-), and para (p-) to describe the relative positions of substituents on a benzene ring:
- Ortho (o-): Substituents are next to each other (1,2- positions).
- Meta (m-): Substituents are separated by one carbon (1,3- positions).
- Para (p-): Substituents are across from each other (1,4- positions)
The IUPAC system is used to name substituted benzenes. The substituents are named as prefixes, and their positions are indicated by numbers. For example, 1,3-dinitrobenzene has two nitro groups at positions 1 and 3 of the benzene ring.
These substituents play a crucial role in the diverse chemistry of aromatic compounds. Whether it’s the electron-donating power of an amino group or the electron-withdrawing strength of a nitro group, each substituent brings its unique flavor to the aromatic party.
Effects of Substituents on Reactivity
- The nature of the substituent significantly influences the reactivity of the benzene ring towards electrophilic substitution reactions.
- EDGs activate the ring, making it more susceptible to electrophilic attack. This is because they increase electron density in the ring, making it more attractive to electrophiles.
- EWGs deactivate the ring, making it less reactive towards electrophilic substitution. They reduce electron density in the ring, making it less attractive to electrophiles.
Other Important Substituents
- Halogens (-F, -Cl, -Br, -I): These are weakly deactivating but ortho/para-directing due to resonance effects.
- Alkyl groups (-CH₃, -C₂H₅): These are weakly activating and ortho/para-directing due to inductive effects.
Effect of Substituents on Orientation in Electrophilic Aromatic Substitution
The presence of a substituent on a benzene ring significantly influences the orientation of the incoming electrophile in subsequent electrophilic substitution reactions. This is primarily due to the electronic effects exerted by the substituent.
Electronic Effects of Substituents
Substituents can be broadly classified into two categories based on their electronic effects:
- Electron-donating groups (EDGs): These groups increase electron density in the benzene ring, making it more reactive towards electrophiles. Examples include -OH, -NH₂, -OCH₃, and alkyl groups.
- Electron-withdrawing groups (EWGs): These groups decrease electron density in the benzene ring, making it less reactive towards electrophiles. Examples include -NO₂, -COOH, -CN, and -SO₃H.
Orientation of Substituents
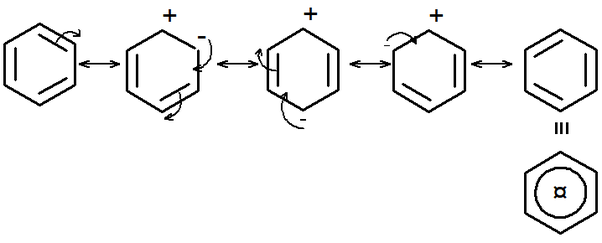
The orientation of the incoming electrophile is determined by the stability of the intermediate carbocation formed during the reaction.
- Ortho-para directors: EDGs tend to direct the incoming electrophile to the ortho and para positions. This is because the positive charge on the intermediate carbocation can be delocalized onto the oxygen or nitrogen atom of the substituent, stabilizing the carbocation.
- Meta directors: EWGs tend to direct the incoming electrophile to the meta position. This is because the positive charge on the intermediate carbocation would be destabilized by being adjacent to the electron-withdrawing group.
Exceptions
Halogens: Although halogens are electron-withdrawing, they are ortho-para directors due to resonance effects. However, they deactivate the benzene ring towards electrophilic substitution.
Resonance and Inductive Effects
The electronic effects of substituents can be further explained by resonance and inductive effects.
- Resonance effect: This involves the delocalization of electrons through the benzene ring. EDGs with lone pairs of electrons (like -OH and -NH₂) can donate electron density to the ring through resonance, increasing electron density at the ortho and para positions.
- Inductive effect: This involves the withdrawal or donation of electrons through the sigma bonds. While both EDGs and EWGs can exert inductive effects, the resonance effect usually dominates in determining the orientation of the incoming electrophile.
The orientation of the incoming electrophile in electrophilic aromatic substitution is primarily determined by the electronic nature of the substituent already present on the benzene ring. EDGs favor ortho and para positions, while EWGs favor the meta position. However, there are exceptions, and both resonance and inductive effects play a role in determining the overall electronic environment of the benzene ring.
Structure and uses of some important benzene derivatives
DDT (Dichlorodiphenyltrichloroethane)
DDT structure
DDT is an organochlorine insecticide that was widely used in the mid-20th century. Its structure consists of two phenyl rings connected by a trichloroethane bridge.
Uses
- Highly effective in controlling insect populations, particularly mosquitoes, which helped in the eradication of malaria and other insect-borne diseases.
- Used in agriculture to protect crops from pests.
Environmental Concerns
Due to its persistence in the environment and bioaccumulation in the food chain, DDT has been banned in many countries. It has been linked to adverse effects on wildlife and human health.
Saccharin
Saccharin structure
Saccharin is an artificial sweetener about 300-400 times sweeter than sucrose. It is a cyclic amide derivative of phthalic acid.
Uses
- Widely used as a sugar substitute for people with diabetes or those looking to reduce calorie intake.
- Added to many food and beverage products to enhance sweetness.
Safety Concerns
While generally considered safe, some studies have raised concerns about potential health risks associated with long-term consumption of saccharin.
BHC (Benzene Hexachloride)
BHC structure
BHC is a mixture of isomers of benzene hexachloride. It is an organochlorine insecticide.
Uses
Used to control a wide range of insects, including pests in agriculture and public health.
Environmental Concerns
Similar to DDT, BHC is persistent in the environment and has been linked to adverse effects on wildlife and human health. Its use has been restricted or banned in many countries.
Chloramine
Structure
Chloramine is not a specific compound but a group of chemical compounds formed by the reaction of ammonia with chlorine. The most common form is monochloramine (NH₂Cl).
Uses
- Widely used as a disinfectant in water treatment to kill bacteria and viruses.
- Used in swimming pools and hot tubs to maintain water hygiene.
Chloramine can cause skin irritation and respiratory problems in some individuals. It is essential to maintain proper chloramine levels in water to ensure safety.
Note: All these compounds have faced regulatory restrictions or bans due to their potential environmental and health hazards. Safer alternatives have been developed and are widely used today.
Summary
Benzene derivatives are compounds formed by replacing one or more hydrogen atoms on the benzene ring with different functional groups. These substituents significantly influence the benzene ring’s reactivity in electrophilic substitution reactions. Electron-donating groups (EDGs) like -OH, -NH₂, and -OCH₃ increase electron density, activating the ring and directing incoming electrophiles to ortho and para positions. Conversely, electron-withdrawing groups (EWGs) like -NO₂, -COOH, and -CN decrease electron density, deactivating the ring and favoring meta substitution. Understanding these effects is crucial for predicting the outcome of reactions involving benzene derivatives. We discussed the structures and applications of DDT, saccharin, BHC, and chloramine. DDT and BHC, once widely used insecticides, have been restricted due to their environmental persistence and harmful effects. Saccharin is an artificial sweetener used as a sugar substitute. Chloramine, a disinfectant, is commonly used in water treatment. While offering benefits, these compounds also pose potential risks, necessitating careful handling and regulation.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us
